Application of FAM127A in pregnancy diseases
A technology of FAM127A and reagents, applied in the field of biomedicine, can solve the problems of slow progress in clinical management and treatment, limited curative effect, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
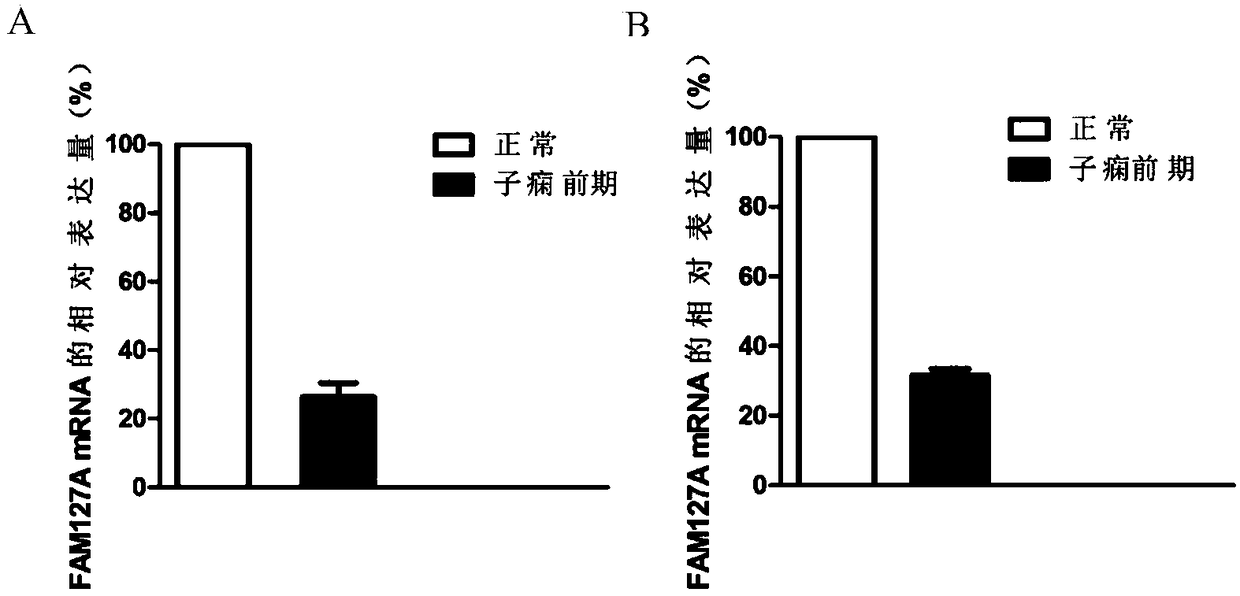
[0068] Example 1 Screening for Gene Markers Related to Preeclampsia
[0069] 1. Sample collection
[0070] 1) Collection of serum samples
[0071] The blood of 45 cases of normal pregnant women and untreated preeclampsia patients was collected, and the EDTA anticoagulant tube was left standing for 10 minutes, and the serum was separated by centrifugation, and stored at -20°C for later use.
[0072] 2) Collection of placenta specimens
[0073] The placenta tissues of 45 cases of preeclampsia and normal pregnant women were collected respectively, rinsed with normal saline twice, after removing water, they were divided into cryopreservation tubes, and stored at -80°C for later use.
[0074] Multiple pregnancies, infectious diseases, chemical drug dependence, maternal smoking, fetal congenital malformations and other pregnancy complications and complications were excluded in both groups. All included subjects signed informed consent before collecting specimens. All the above sp...
Embodiment 2
[0089] Example 2 QPCR sequencing to verify the differential expression of the FAM127A gene
[0090] 1. Large-sample QPCR verification of differential expression of FAM127A gene.
[0091] 2. RNA extraction
[0092] Use QIAGEN tissue RNA extraction kit to extract total RNA in placental tissue, and blood RNA extraction kit to extract RNA in serum. For specific steps, refer to the instruction manual.
[0093] 3. Reverse transcription:
[0094]1) Add dNTP mixture 1μl, Oligo dT primer 1μl, total RNA 2μg, add RNase FreeddH 2 O Make the total volume to 10 μl, carry out denaturation and annealing reaction on the PCR instrument, 65°C, 5min, place at 4°C after the reaction is completed.
[0095] 2) Construct a 20 μl reaction system, continue to add 4 μl of 5×Primer Script Buffer, 0.5 μl of RNase Inhibitor, 0.5 μl of Prime Script RTase, RNase Free ddH 2 O 5.0 μl, carry out the reverse transcription reaction on the PCR instrument according to the following conditions: 42°C for 15-30 mi...
Embodiment 3
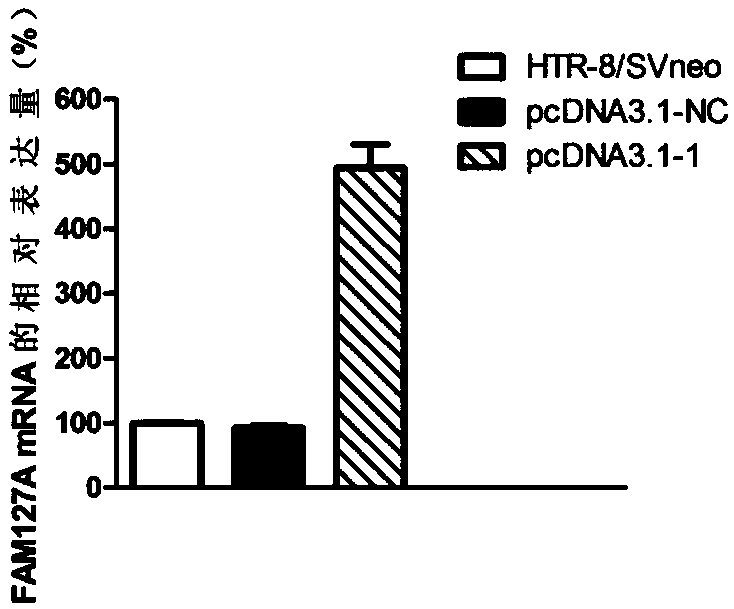
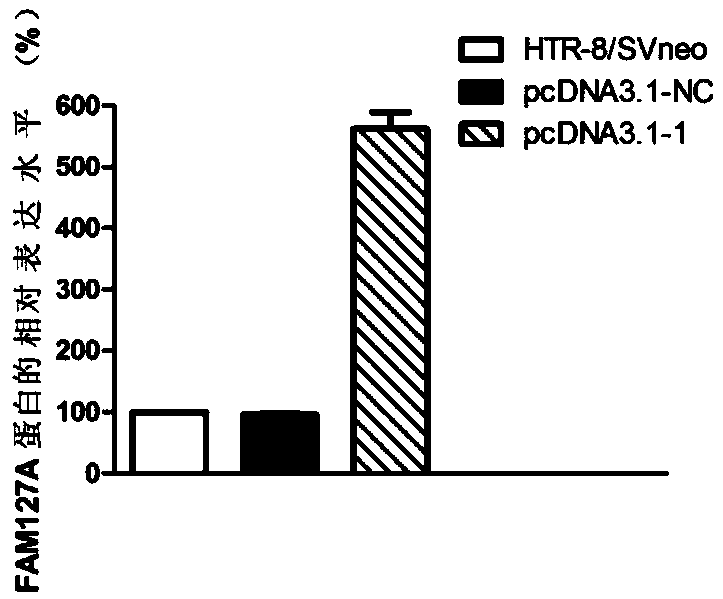
[0113] Overexpression of embodiment 3FAM127A gene
[0114] 1. Cell culture
[0115] Human early pregnancy villous trophoblast cell line (HTR-8 / SVneo) was incubated in RPIM-1640 medium containing 10% fetal bovine serum at 37°C, 5% CO 2 cultured in an incubator. Change the medium once every 2-3 days, and use 0.25% EDTA-containing trypsin for routine digestion and passage.
[0116] 2. Transfection
[0117] 1) Treatment of cells before transfection
[0118] The trophoblast HTR-8 / SVneo in the logarithmic phase was treated with 1×10 5 The densities were planted in six-well plates at 37°C in 5% CO 2 cultured in an incubator.
[0119] 2) Construction of gene overexpression vector
[0120] Synthesize specific PCR amplification primers according to the sequence of FAM127A in GeneBank, and the primer sequences are as follows:
[0121] Forward primer: 5'-CCGGAAGCTTGCCACCATGGACGGTCGGGTGC-3' (SEQ ID NO.5)
[0122] Reverse primer: 5'-CGGGCGGCCGCGAAGTCCTCGTCTCTCCTCCCA-3' (SEQ ID NO.6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com