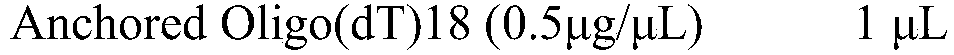DOCK10 gene for early diagnosis of myocardial infarction
A DOCK10, myocardial infarction technology, applied in disease diagnosis, microbial determination/examination, biochemical equipment and methods, etc., can solve the problem of unclear expression of related genes, achieve early diagnosis, reduce mortality, gene more effective diagnosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
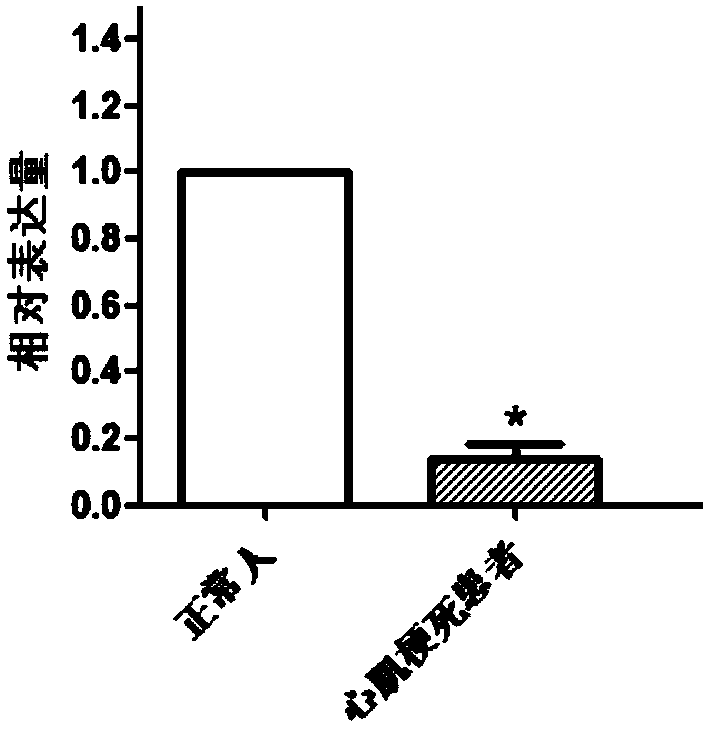
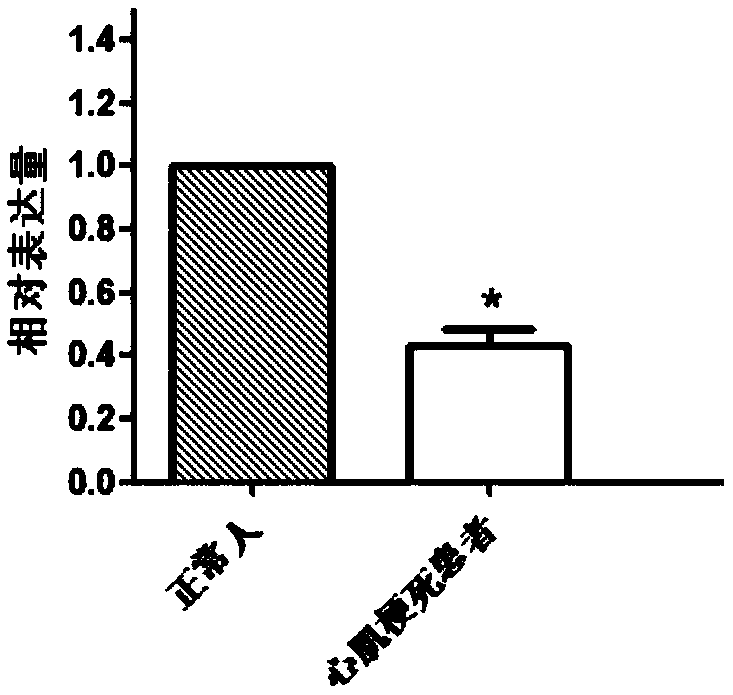
[0034] Example 1 Screening of genes differentially expressed in patients with myocardial infarction and normal people
[0035] 1. Research object
[0036] Select 6 hospitalized patients with myocardial infarction treated in the hospital as research objects, including 3 males and 3 females, with an average age of 56.00±8.75; 7 healthy people in the control group; each of the above-mentioned patients and healthy people who were invited to participate in the study All signed the informed consent.
[0037] 2. Sample collection and storage
[0038] On the day of admission, 8 mL of fresh sterile arterial blood was collected into EDTA anticoagulated purple tubes before coronary angiography. If it is not used immediately, the sample can be stored in a refrigerator at 4°C for 2 hours.
[0039] 3. Separation of PBMCs by Ficoll method
[0040] The following steps are all completed in the ultra-clean bench:
[0041] (1) Dilute the blood sample with equal volume of normal saline, add ...
Embodiment 2
[0063] Embodiment 2QPCR experiment verifies the genes differentially expressed in patients with myocardial infarction and normal people
[0064] 1. Research object:
[0065] The screening criteria were the same as in Example 1, 35 patients with myocardial infarction and 35 normal subjects.
[0066] 2. Extraction of total RNA in blood
[0067] Step is with embodiment 1.
[0068] 3. RT-PCR
[0069] (1)RT
[0070] RT reaction system (20μl):
[0071]
[0072]
[0073] RT reaction procedure:
[0074] 42°C 15min
[0075] 85℃ 5s
[0076] 4℃ ---
[0077] (2) qPCR
[0078] PCR reaction system (20μl):
[0079]
[0080] PCR reaction program:
[0081] Amplification procedure:
[0082] Stage 1 95°C 10min
[0083] Stage 2 95°C 10s
[0084] 55℃ 10s
[0085] Repeat 40 cycles
[0086] Three replicate wells were set for each sample, and the internal reference was GAPDH.
[0087] (3) Primers
[0088] The primer sequences of DOCK10 gene and GAPDH gene are as follows:
[...
Embodiment 3
[0102] Example 3 Western blot experiment to verify the expression products of differentially expressed genes in patients with myocardial infarction and normal people
[0103] 1. Research object: same as embodiment 2.
[0104] 2. Mononuclear cell isolation
[0105] Take 10ml of venous blood from patients with myocardial infarction and normal people, inject it into a sterile vial containing heparin, and shake it gently immediately after capping. Add an equal volume of HBSS (NaCl 8.0g, NaCl 2 HPO 4 0.132g, KH 2 PO 4 0.06g, KCl0.4g, phenol red 1ml, NaHCO 3 0.35g, D-glucose 1.0g, dissolved in 1000ml double distilled water), to reduce the aggregation of red blood cells. Draw 8ml of lymphocyte stratification solution into a 50ml centrifuge tube, slowly add the diluted blood along the tube wall, keep the interface clear, do not mix the two, centrifuge at 20°C 2000r / min for 30min, carefully absorb the stratification solution and plasma transfer The turbid off-white layer, tha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com