Medicinal composition and application thereof
A composition and drug technology, applied in the field of medicine, can solve problems such as complex efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The preparation of embodiment 1 composition of the present invention
[0032] Each component was weighed according to the weight percentage in Table 1 below, and mixed uniformly to obtain compositions 1, 2 and 3, respectively.
[0033] Composition of Table 1 Compositions 1 to 3
[0034]
[0035] Among them, the preparation method of the total ginseng saponins extract is as follows: take red ginseng as raw material, add 75% ethanol solution for reflux extraction after crushing, the mass volume ratio of red ginseng to ethanol is 1:20g / mL, combine the extracts, concentrate ; The concentrated solution is purified by D101 macroporous resin to obtain total ginsenoside extract.
[0036] The composition of the total ginseng saponins extract obtained is analyzed, and the results are as follows in Table 2:
[0037] The composition of table 2 ginseng total saponins extract
[0038]
Embodiment 2
[0039] The safety evaluation of embodiment 2 compositions of the present invention
[0040] The research of the nonclinical safety evaluation of composition of the present invention is as follows:
[0041] 1. Oral acute toxicity test in mice
[0042] Under the conditions of maximum administration concentration and maximum administration capacity, mice were orally administered compositions 1 to 3 of the present invention, observed continuously for 14 days, and then the maximum tolerated doses of compositions 1 to 3 of the present invention were all 10 mg. / kg.
[0043] 2. Beagle dog oral acute toxicity test
[0044] Under the conditions of maximum dosage concentration and maximum dosage volume, Beagle dogs were orally administered compositions 1-3 of the present invention, and then the maximum tolerated dose of the composition of the present invention was all 10 mg / kg.
[0045] 3. Long-term toxicity of intragastric administration in rats
[0046] Compositions 1 to 3 of the ...
Embodiment 3
[0053] Embodiment 3 Pharmacodynamics
[0054] Ginsenoside monomer alleviates the development of oral ulcers in Hamster mice
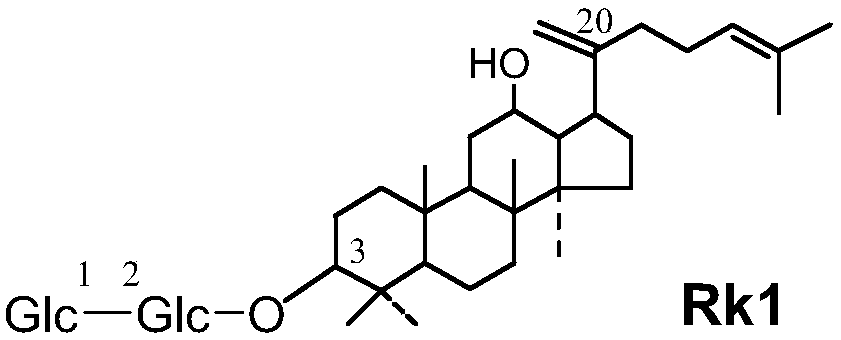
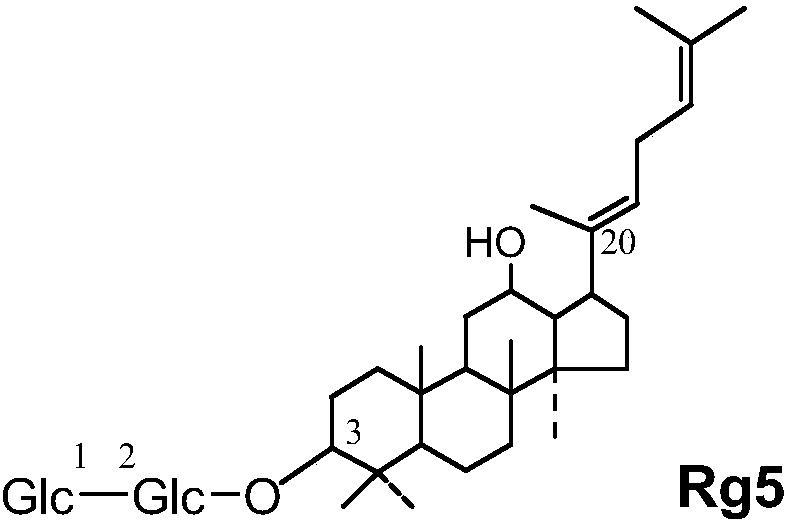
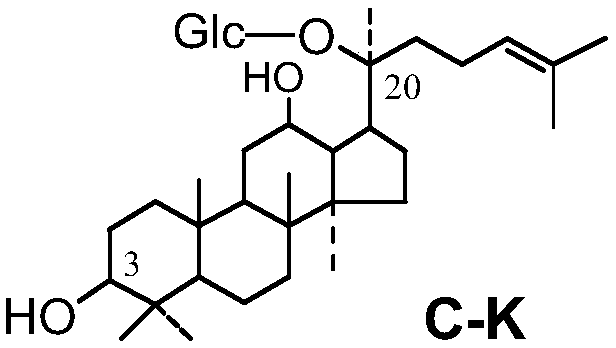
[0055] Materials: Hamster mouse, 5'fluorouracil (Sigma), ginsenoside Rk1, ginsenoside Rg5, ginsenoside C-K, ginsenoside Rg3, ginsenoside Rh4, composition 1-3 obtained in Example 1, total ginsenoside extract . 5'fluorouracil was prepared with physiological saline to prepare a solution with a final concentration of 10 mg / mL, and the five kinds of saponin monomers, ginseng total saponin extracts, and compositions 1-3 were respectively prepared with physiological saline with a final concentration of 5 mg / mL.
[0056] Grouping of mice: Divide the mice into 10 groups, 10 in each group, half male and half male, respectively: ginsenoside Rk1 group, ginsenoside Rg5 group, ginsenoside C-K group, ginsenoside Rg3 group, ginsenoside Rh4 group, combination Group 1, group 2, group 3, group ginsenosides extract, and control group.
[0057] Methods: Seven-week-old Ha...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Maximum tolerated dose | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com