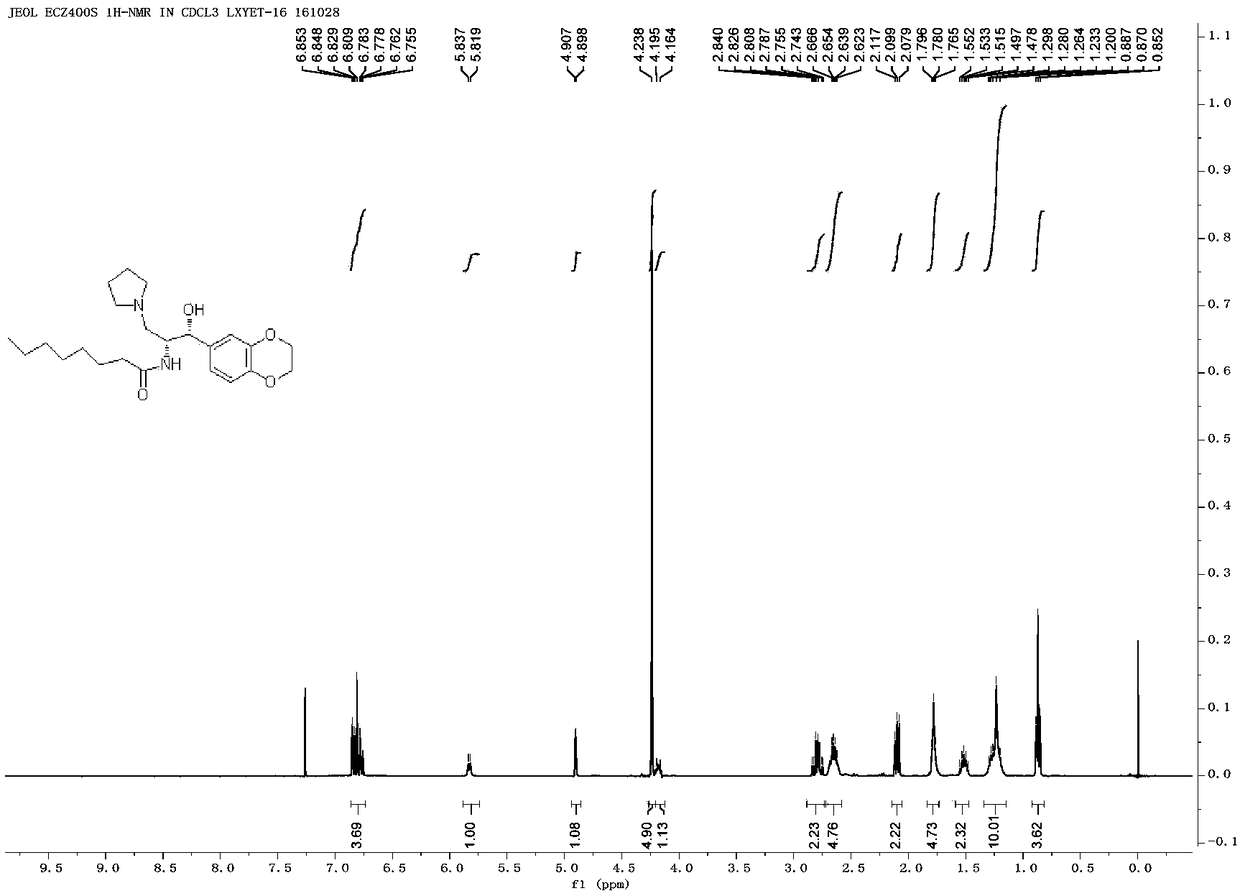
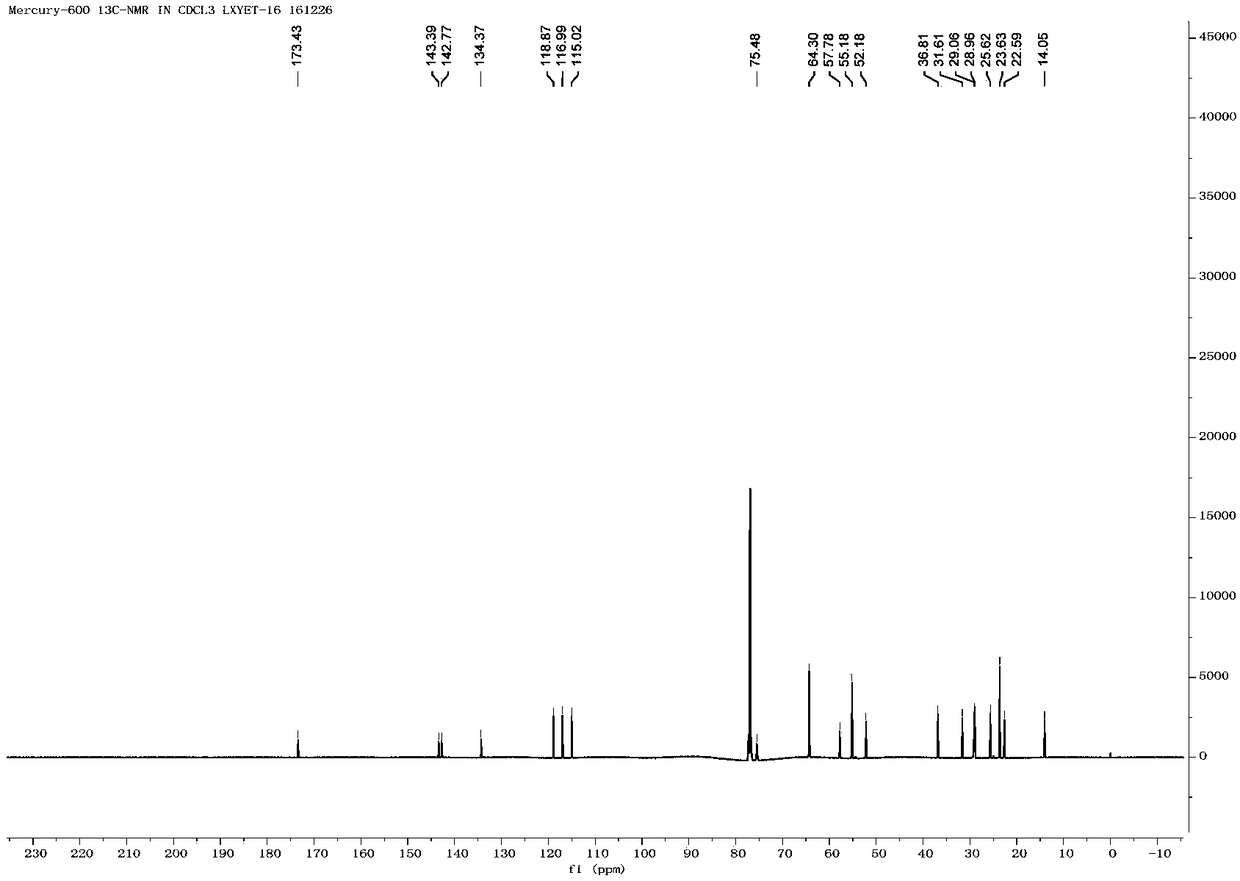
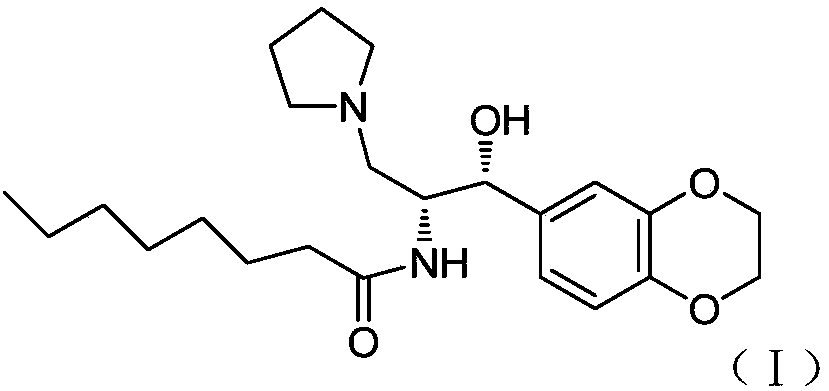
Method for preparing Eliglustat
A compound and reaction technology, which is applied in the field of preparation of iligrustat and glucosylceramide synthase inhibitors, can solve the problems of inability to apply large-scale industrial production, difficult preparation, and difficult preparation of catalysts, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] The preparation of embodiment 1 formula (X) compound
[0051] Add formula (X) compound (4.47g, 25mmol), azidoacetic acid (3g, 30mmol), dicyclohexylcarbodiimide (5.7g, 27mmol) and 4-dimethylamino in a 250mL round bottom flask at 0°C Pyridine (370mg, 3.0mmol), dichloromethane 100mL, warmed to room temperature after 30min, reacted for 1h, filtered through silica gel, washed with cyclohexane: ethyl acetate = 7:3, concentrated the mother liquor to obtain the crude product, and then removed it with a short column. 6.3 g of the product was obtained with a yield of 97%.
[0052] [α]20D+165(c,1.0,CHCl 3 ); 1 H NMR (400MHz, CDCl 3 )δ7.3-7.43 (m, 5H), 5.71 (dd, J = 3.2Hz, 8.4Hz, 1H), 4.81-4.94 (m, 3H), 4.54-4.57 (m, 1H); 13 C NMR (150MHz, CDCl 3 )δ184.8, 168.4, 137.8, 129.2, 126.2, 75.1, 62.2, 54.6.
Embodiment 2
[0053] The preparation of embodiment 2 formula (XII) compound
[0054] Add formula (XI) compound (4.8g, 25mmol), azidoacetic acid (3g, 30mmol), dicyclohexylcarbodiimide (5.7g, 27mmol), 4-dimethylamino in a 250mL round bottom flask at 0°C Pyridine (370 mg, 3.0 mmol), dichloromethane 100mL, rise to room temperature after 30min, react for 1h, filter on silica gel, wash with cyclohexane: ethyl acetate = 7:3, concentrate the crude product of the mother liquor, and then wash with dichloromethane / petroleum ether recrystallization to obtain 6.5g of product, yield 94.2%.
[0055] [α]20D+310(c,1.0,CHCl 3 ); 1 H NMR (400MHz, CDCl 3 )δ7.25-7.42(m,5H),6.25(d,J=9.2Hz,1H),4.74-4.95(m,2H),4.04(dd,J=11.6Hz,8.4Hz,1H),3.17( dd,J=1.2Hz,11.2Hz,1H); 13 C NMR (150MHz, CDCl 3 )δ202.0, 168.7, 138.4, 129.1, 128.8, 125.4, 69.5, 55.3, 37.6.
Embodiment 3
[0056] The preparation of embodiment 3 formula (Ⅲ) compound
[0057] Formula (II) compound (5mmol) in 50mL dichloromethane, drop TiCl at -78°C 4 (0.575mL, 5.25mmol), reacted at this temperature for 15min, then added DIPEA (0.96mL, 5.5mmol) and stirred for 1h, then NMP (0.96mL, 10mmol), stirred for 15min and added 1,4-benzodiox alkane-6-carboxaldehyde, and stirred at this temperature for 40min, then warmed up to -30°C for 40min, after TLC showed that it was complete, the reaction was terminated with saturated ammonium chloride (20mL), extracted with 150mL ethyl acetate, washed with saturated sodium chloride, no Dry over sodium sulfate, filter, evaporate the solvent, recrystallize after desalting with a short column of silica gel to obtain 1.2 g of the oxazolidinone product, with a yield of 79%; 1.7 g of the thiazolidinone product, with a yield of 77%.
[0058] Oxazolidinone Products:
[0059] [α]20D+39(c,1.0,CHCl 3 ); 1 H NMR (400MHz, CDCl 3 )δ7.27-7.42(m,5H),6.86-7.02(m,3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com