Genetically engineered cell and method for efficiently amplifying NK cells in vitro
A genetic engineering, NK cell technology, applied in the field of immunology, can solve the problems of uncertainty of NK cell culture, complex animal serum composition, introduction of mycoplasma, etc., to achieve the effect of low cost, large quantity, and enhanced lethality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: Preparation of genetically engineered cells IL-15-IL-18-4-1BBL-K562 cells
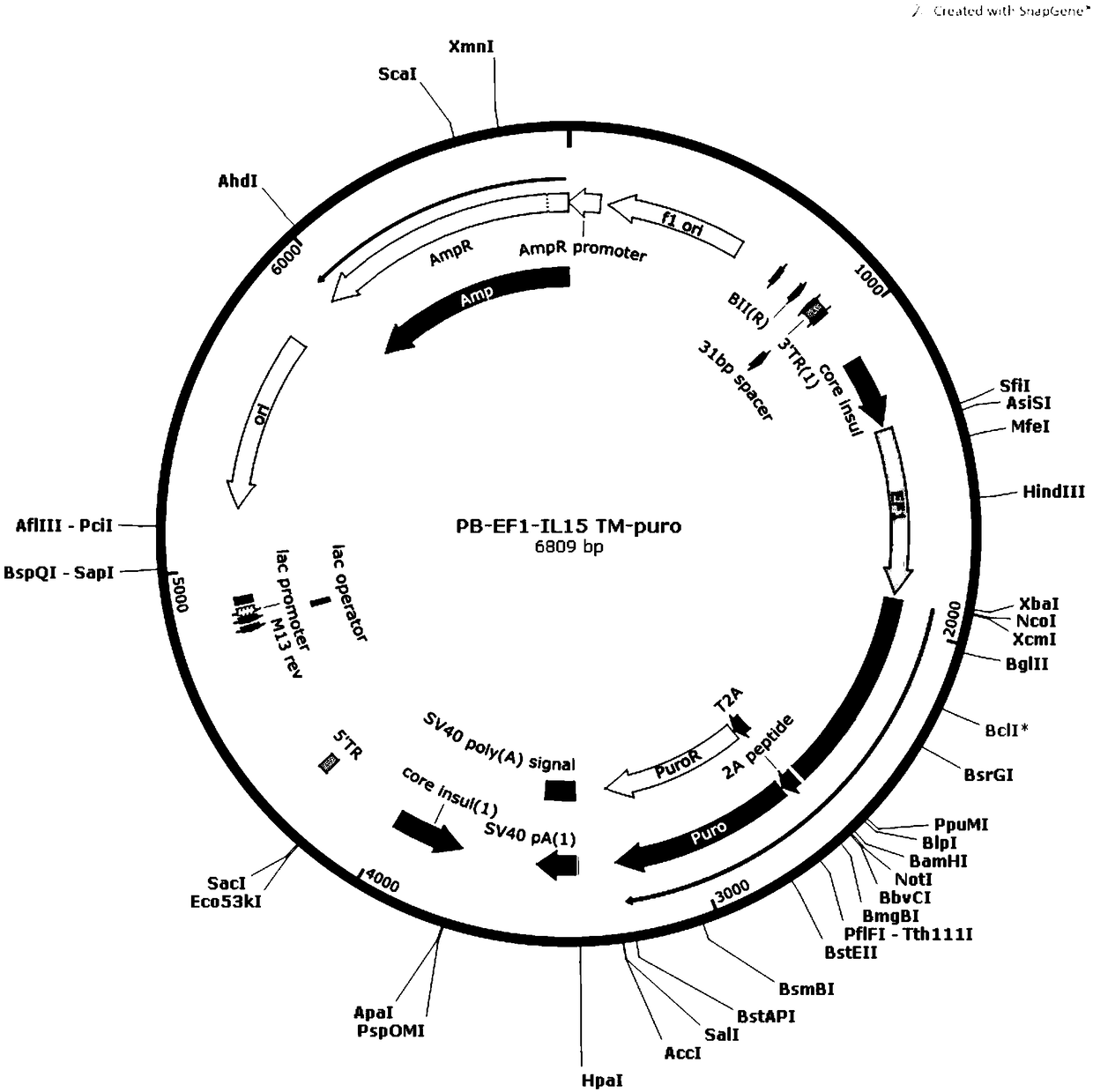
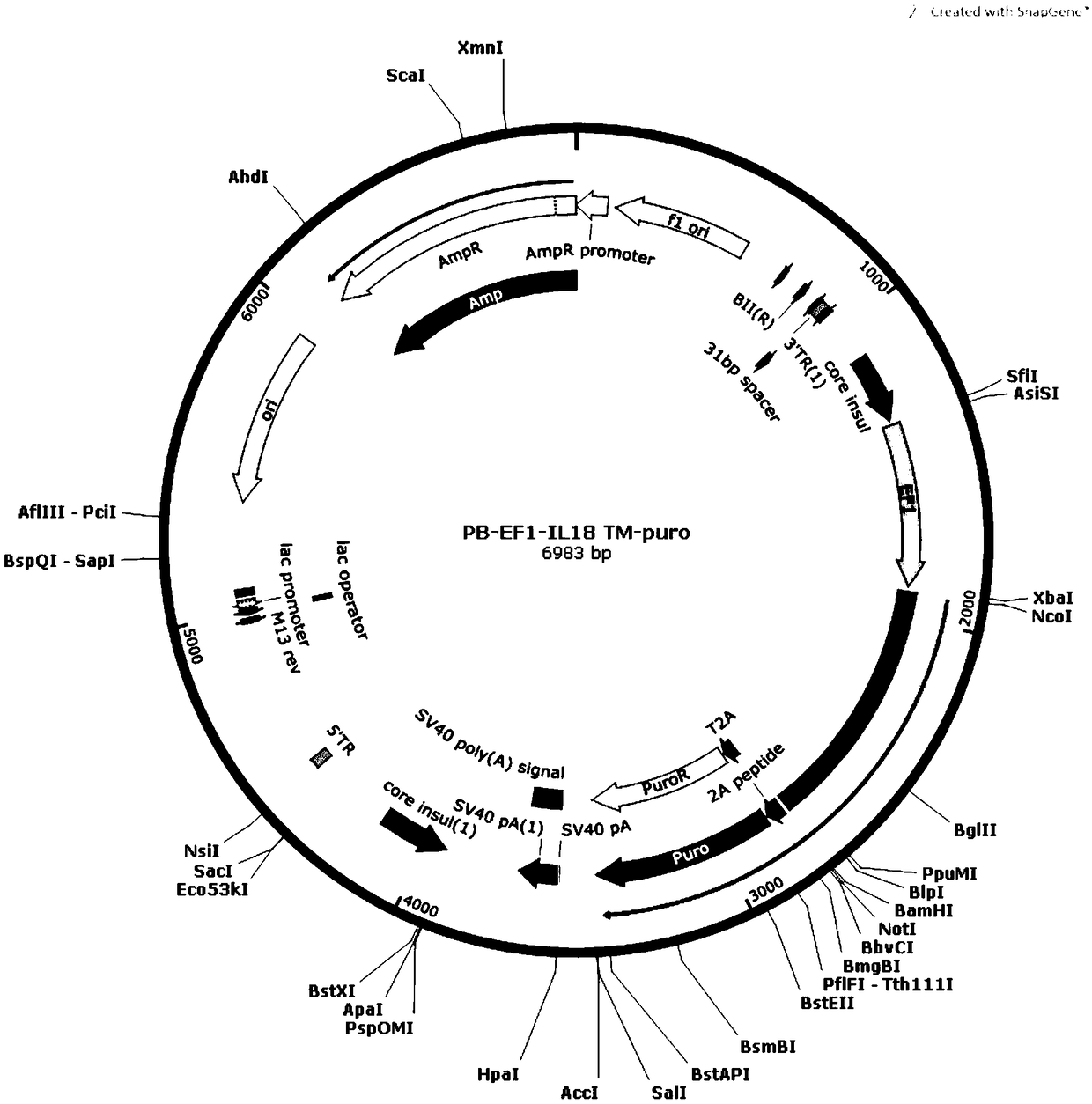
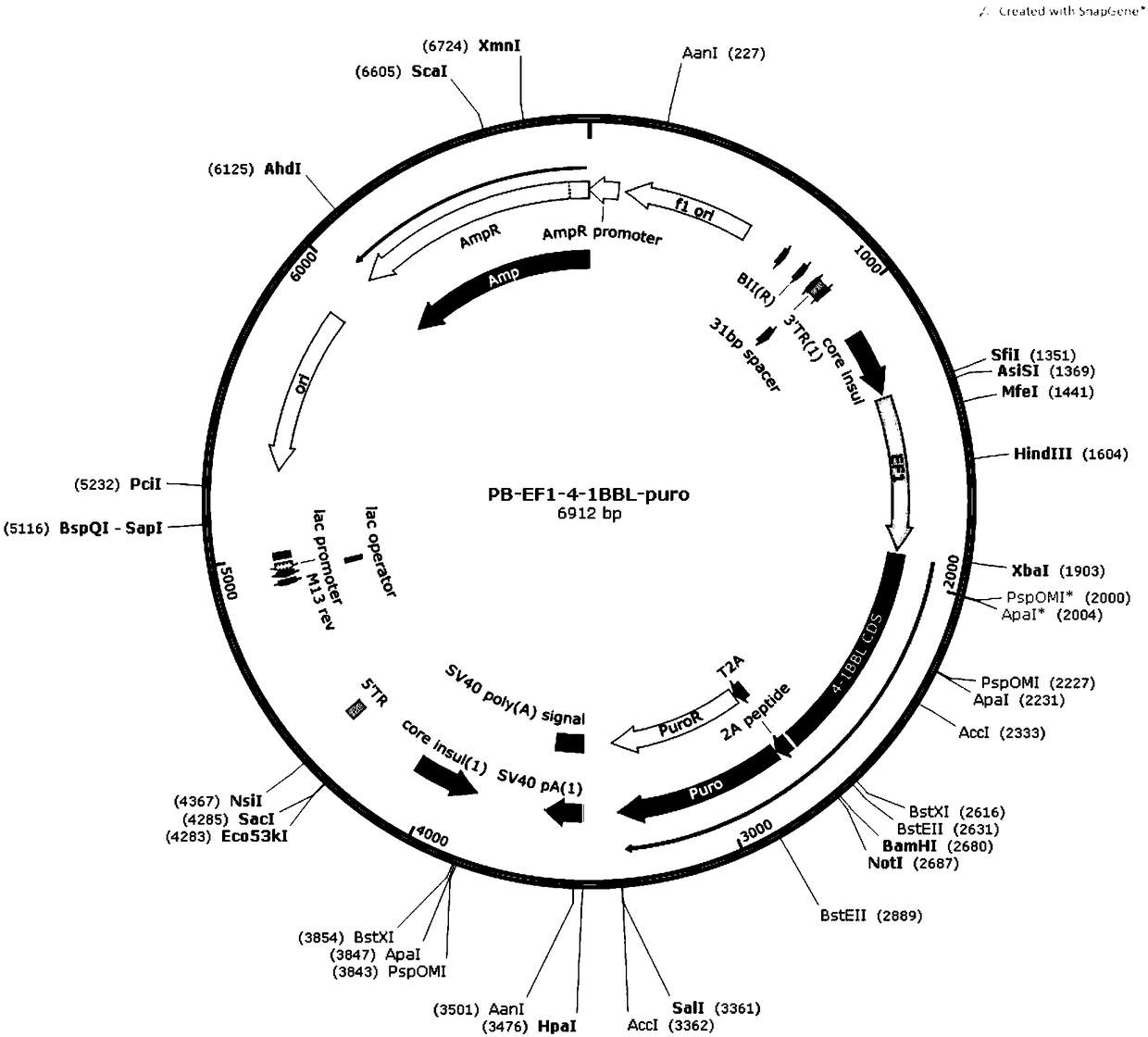
[0041] (1) The IL-15, IL-18, and 4-1BBL genes were amplified by PCR, and then the respective amplification products were inserted into the XbaIBamHI site of the Piggybac Sleeping Beauty expression vector to construct PB-EF1-IL-15TM respectively -puro, PB-EF1-IL18TM-puro, PB-EF1-4-1BBL-puro transposons.
[0042] (2) Co-transfect K562 cells with PB-EF1-IL-15TM-puro transposon and transposase, and culture the transfected K562 cells; sort by flow cytometry to obtain IL-15 transmembrane Stably expressed K562 cells (IL-15-K562 cells).
[0043] (3) Co-transfect IL-15-K562 cells with PB-EF1-IL-18TM-puro transposon and transposase, and culture the transfected K562 cells; sort by flow cytometry to obtain IL -15. K562 cells stably expressing IL-18 across the membrane (IL-15-IL-18-K562 cells).
[0044] (4) Co-transfect IL-15-IL-18-K562 cells with PB-EF1-4-1BBL-puro transposon and transposase T...
Embodiment 2
[0045] Example 2: In vitro efficient expansion of NK cells
[0046] 1. Preparation of PBMC cells
[0047] Use an anticoagulant tube to collect peripheral blood from healthy people, and shake it while collecting to fully mix the peripheral blood with the anticoagulant; slowly add the anticoagulant blood to a 50ml centrifuge tube filled with an equal volume of lymphocyte separation medium (Ficoll), 450g, Centrifuge slowly for 25 minutes, and do not stop the centrifugation in the middle; after the centrifugation, carefully absorb the buffy coat cells above the lymphocyte separation solution, transfer to a new 50ml centrifuge tube, add PBS, 300g, and centrifuge slowly for 10 minutes , discard the supernatant, and keep the cell pellet at the bottom of the centrifuge tube; add PBS again, 160g, centrifuge slowly for 15min, discard the supernatant; finally add PBS, 300g, centrifuge slowly for 10min, discard the supernatant, and obtain PBMC cell.
[0048] 2. Expansion and culture of ...
Embodiment 3
[0057] Example 3: Determination of NK cell killing activity
[0058] The killing effect of NK cells on human leukemia cells K562 was detected by 4h LDH release assay.
[0059] 1. Take the passaged cell line K562 cells and count them to make 1×10 5 cells / mL cell suspension, add to 96-well plate, 50μl per well.
[0060] 2. Add the cultured NK cells into the 96-well plate according to the effect: target ratio of 1:1, 10:1, 20:1, and 40:1. At the same time, set the natural release holes for effector cells and target cells, and the natural release holes for the medium , target cell maximum release well, volume correction control, each well volume is 100μl, set 3 duplicate wells, centrifuge at 250g for 4min, place at 37°C, 5% CO 2 , 95% saturated humidity incubator for 4 hours.
[0061] 3. 45 minutes before the end of the reaction, add 10 μl of lysate to each well of the target cell maximum release well. After the reaction, pipette 50 μl supernatant and 50 μl LDH enzyme reaction...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com