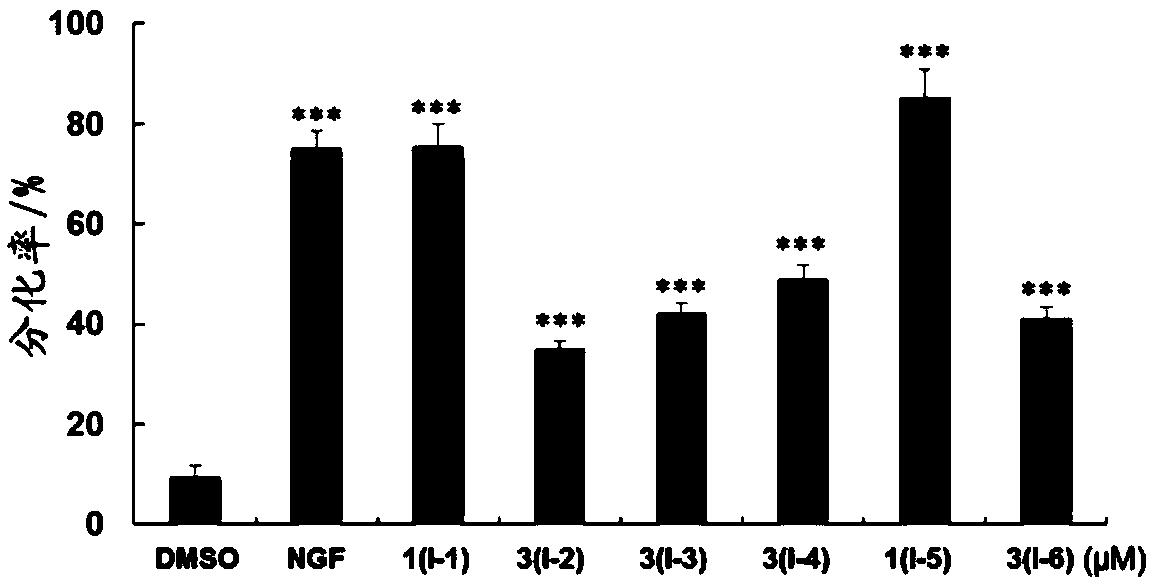
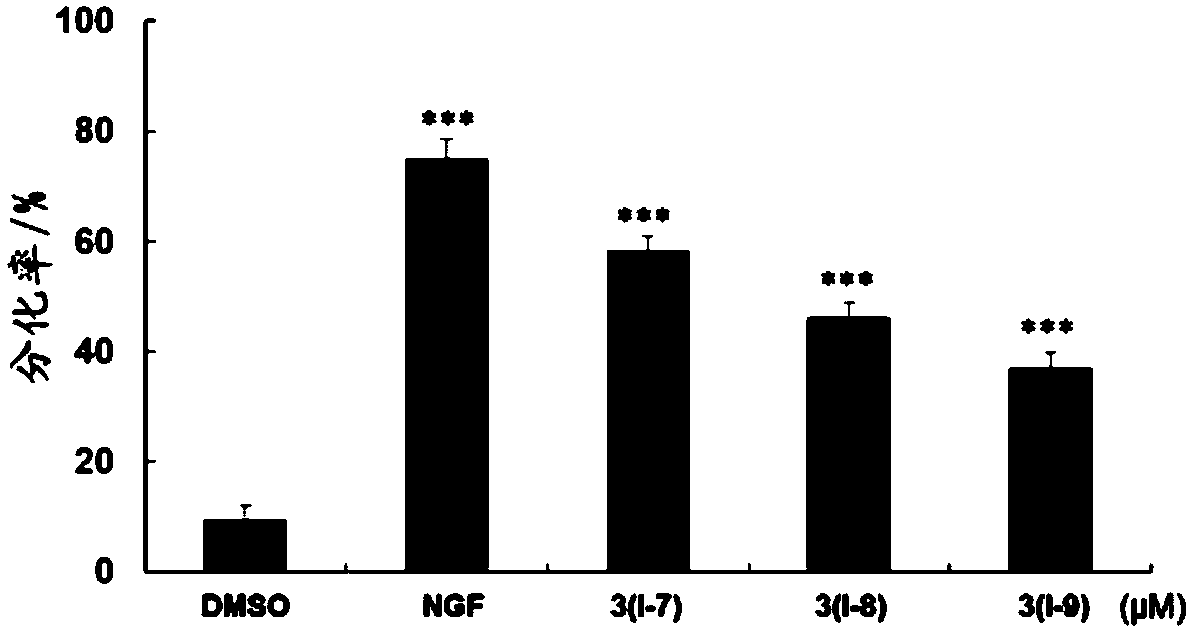
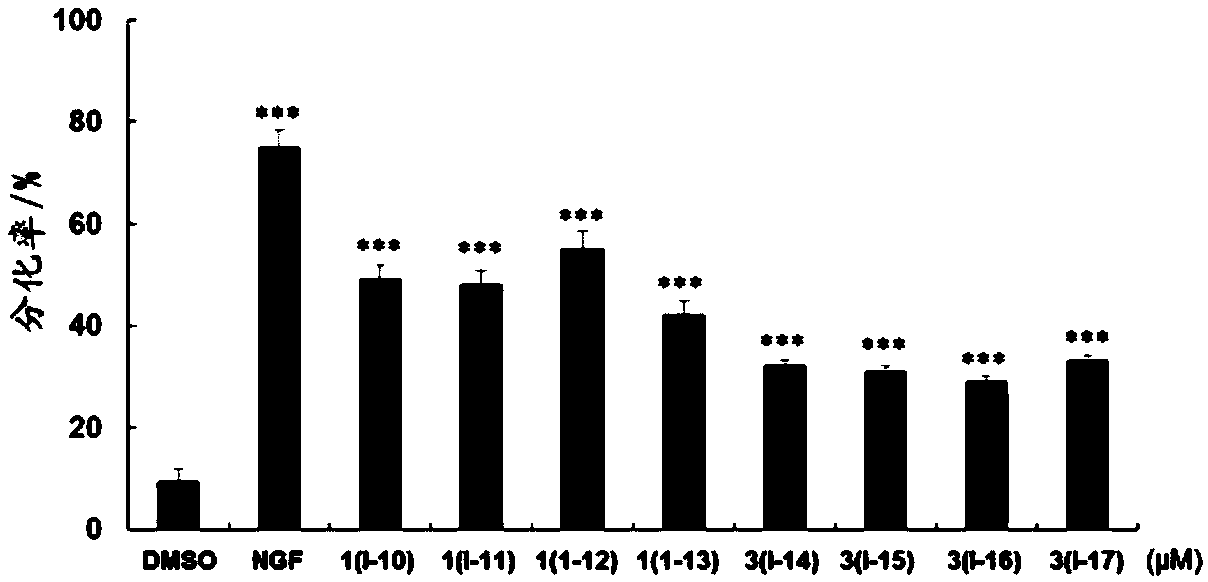
Pentacyclic triterpenoid compound, derivatives of pentacyclic triterpenoid compound and applications
A technology for pentacyclic triterpenoids and compounds, which is applied in the field of chemical pharmacy, can solve the problems of large molecular weight, difficult to prepare on a large scale, and no treatment method has been found, and achieves the effects of improving cognition and high activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Compound Ⅰ-1: (3S,4R)-23,28-Dihydroxyolean-12-en-3-yl(2E)-3-(3,4-dihydroxyphenyl)acrylate
[0044] Caffeic acid (A) (408mg, 1mmol), DCC (413mg, 2mmol) and DMAP (122mg, 1.0mmol) protected by phenolic hydroxyl group O-TBS were dissolved in 10mL of anhydrous dichloromethane, cooled to 0°C, and two 3β,23,28-Triol olean-12-ene (B) (34.3 mg, 0.05 mmol) protected by O-TBS with a primary hydroxyl group, and the reaction system was stirred overnight at room temperature. After purification, 30 mg of colorless solid C was obtained, yield: 56%. The compound structure is shown below:
[0045]
[0046] Compound C (21.5 mg, 0.02 mmol) was dissolved in 5 mL of methanol, and camphorsulfonic acid (23 mg, 0.1 mmol) was added. The reaction system was stirred at room temperature for 4 hours. Quenched with water, extracted with ethyl acetate, washed the organic phase with saturated brine, dried over anhydrous sodium sulfate, filtered, and concentrated by rotary evaporation to obtain a ...
Embodiment 2
[0051] Compound Ⅰ-2: (3S,4R)-23,28-Dihydroxyolean-12-en-3-yl(2E)-3-phenylacrylate;
[0052] The synthesis method is the same as compound Ⅰ-1, and the reaction materials are: cinnamic acid (1 mmol), DCC (2 mmol), DMAP (1.0 mmol) and compound B (0.05 mmol). 0.02 mmol of the obtained product was dissolved in 5 mL of methanol, and 0.05 mmol of camphorsulfonic acid was added.
[0053] 1 H NMR (500MHz, CDCl 3 ):7.65(d,J=16.0Hz,1H),7.62(m,2H),7.43(m,3H),6.53(d,J=16.0Hz,1H),5.23(t,J=3.5Hz,1H ),5.03(dd,J=11.5.0,6.0Hz,1H),3.56(d,J=11.0Hz,1H),3.39(d,J=12.0Hz,1H),3.19(d,J=11.0Hz ,1H),3.14(d,J=12.0Hz,1H),1.25(s,3H),1.09(s,3H),1.04(s,3H),0.92(s,6H),0.87(s,3H) . 13 C NMR (125MHz, CDCl 3 )167.8,145.8,141.8,134.5,130.3,128.9,128.1,123.4,117.9,75.8,69.8,64.7,49.1,47.9,47.8,43.8,43.4,43.0,41.2,39.2,38.1,37.331.3,38,3,3 ,32.3,31.8,26.6,26.6,24.6,24.1,23.5,22.9,18.8,17.3,16.5,13.9.HRMS:m / z[M+Na] + calcd for C 39 h 56 o 4 Na:611.4071,found:611.4063.
[0054] The structural formula of th...
Embodiment 3
[0057] Compound Ⅰ-3: (3S,4R)-23,28-Dihydroxyolean-12-en-3-yl(2E)-3-(3-hydroxyphenyl)acrylate;
[0058] The synthesis method is the same as compound Ⅰ-1, and the reaction materials are: 3-hydroxycinnamic acid (1 mmol) protected by phenolic hydroxyl O-TBS, DCC (2 mmol), DMAP (1.0 mmol) and compound B (0.05 mmol). 0.02 mmol of the obtained product was dissolved in 5 mL of methanol, and 0.07 mmol of camphorsulfonic acid was added.
[0059] 1 H NMR (500MHz, CDCl 3 ):7.63(d,J=16.0Hz,1H),7.23(t,J=8.0Hz,1H),7.05(d,J=8.0Hz,1H),7.00(t,J=2.5Hz,1H), 6.85(dd, J=8.0,2.5Hz,1H),6.47(d,J=16.0Hz,1H),5.21(t,J=3.5Hz,1H),5.02(dd,J=11.0,6.0Hz,1H ),3.54(d,J=11.0Hz,1H),3.38(d,J=11.5Hz,1H),3.18(d,J=11.0Hz,1H),3.11(d,J=11.5Hz,1H), 1.13(s,3H),1.02(s,3H),0.99(s,3H),0.88(s,3H),0.87(s,3H),0.86(s,3H). 13 C NMR (125MHz, CDCl 3 )168.2,159.5,146.3,145.8,136.1,131.5,123.4,121.9,119.2,118.1,115.5,75.1,69.9,65.2,49.1,47.3,46.9,43.8,43.1,42.5,41.6,33.2,378.3,38.3 ,33.3,33.1,32.4,31.8,26.4,26.4,24.6,24.1,24....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com