Synthesis method of hyperbranched polyester
A technology of hyperbranched polyester and synthesis method, applied in the field of polymer chemistry, can solve the problems of difficulty in achieving better use effect, high cost, and reducing the surface effect of polyester resin.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
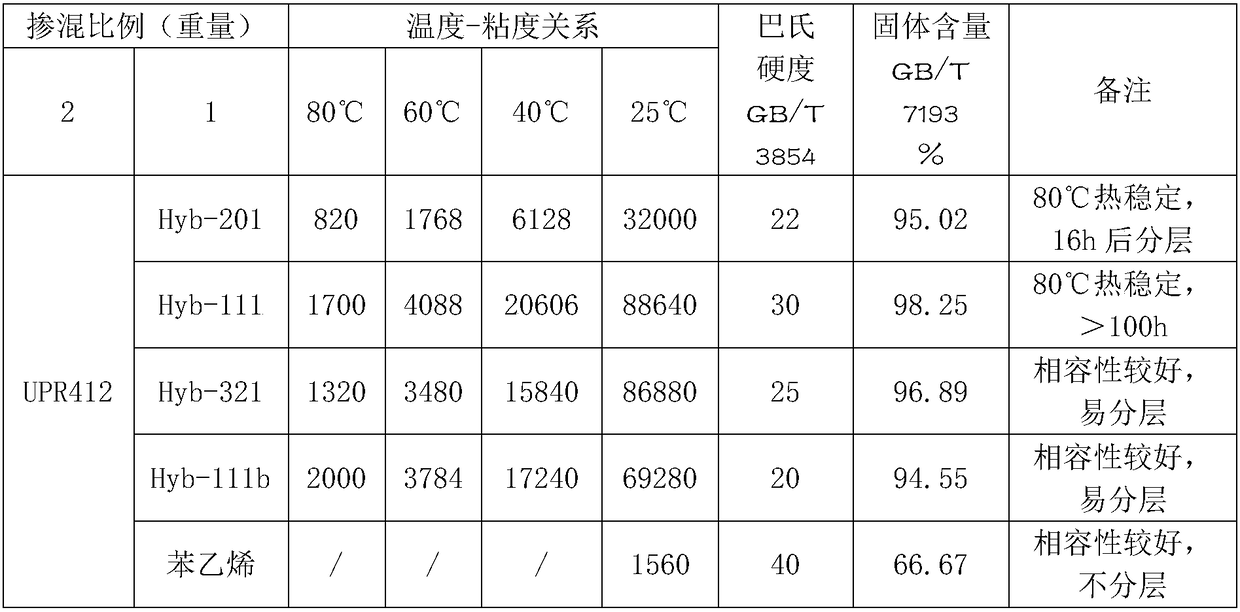
[0036] Example 1 - the first generation resin Hyb-201
[0037] In a 2L four-neck flask equipped with a thermometer, a mechanical stirrer, a nitrogen inlet tube and a reflux condenser, add 134.2g trimethylolpropane, 402.4g 2,2-dimethylolpropionic acid, and 26.8g xylene in sequence , 1.1g of p-toluenesulfonic acid and 0.7g of TPP triphenyl phosphite as an auxiliary agent, turn on mechanical stirring, heat up to 140°C for 1h under nitrogen protection, and keep warm for reaction until the acid value is lower than 20mgKOH / g, then cool down to 110°C, add 801.3g lauric acid, 40.1g xylene, 1.60g p-toluenesulfonic acid and 1.04g TPP triphenyl phosphite, heat up to 160°C for 1 hour, then gradually raise the temperature to 180°C for heat preservation reaction, the reaction When the acid value is 25-30mgKOH / g, cool down to 110°C, add 172.3g methacrylic acid, 8.6g xylene, 0.35g p-toluenesulfonic acid and 0.25g TPP triphenyl phosphite, heat up to 160°C for 1 hour , and gradually raise the ...
Embodiment 2
[0038] Embodiment 2-the first generation hyperbranched unsaturated polyester resin Hyb-111
[0039]In a 2L four-neck flask equipped with a thermometer, a mechanical stirrer, a nitrogen inlet tube and a reflux condenser, add 134.2g trimethylolpropane, 402.4g 2,2-dimethylol propionic acid, 26.8g di Toluene, 1.1g of p-toluenesulfonic acid and 0.7g of TPP triphenyl phosphite as an auxiliary agent, turn on the mechanical stirring, heat up to 160°C for 1h under the protection of nitrogen for reaction, keep the reaction until the acid value is lower than 20mgKOH / g, then lower the temperature When the temperature is lower than 140°C, add 400.6g lauric acid, 228.2g caprolactone, 31.5g xylene, 1.25g p-toluenesulfonic acid and 0.82g TPP triphenyl phosphite, heat up to 160°C for 1 hour, and gradually Heat up to 180°C for heat preservation reaction, react until the acid value is 25-30mgKOH / g, cool down to 110°C, add 172.3g of methacrylic acid, 8.6g of xylene, 0.35g of p-toluenesulfonic aci...
Embodiment 3
[0040] Embodiment 3-the first generation hyperbranched unsaturated polyester resin Hyb-321
[0041] In a 2L four-neck flask equipped with a thermometer, a mechanical stirrer, a nitrogen inlet tube and a reflux condenser, add 134.2g trimethylolpropane, 402.4g 2,2-dimethylolpropionic acid, and 26.8g xylene in sequence , 1.1g of p-toluenesulfonic acid and 0.7g of TPP triphenyl phosphite as an auxiliary agent, turn on mechanical stirring, heat up to 180°C for 1h under nitrogen protection, and keep warm for reaction until the acid value is lower than 20mgKOH / g, then cool down to 110°C, add 600.9g of lauric acid, 228.2g of caprolactone, 35.6g of xylene, 1.43g of p-toluenesulfonic acid and 0.93g of TPP triphenyl phosphite, raise the temperature to 160°C and keep it for 1h, then gradually raise the temperature to 180°C Carry out heat preservation reaction, react until the acid value is 25-30mgKOH / g, cool down to 110°C, add 72.1g of acrylic acid, 4.3g of xylene, 0.17g of p-toluenesulfo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com