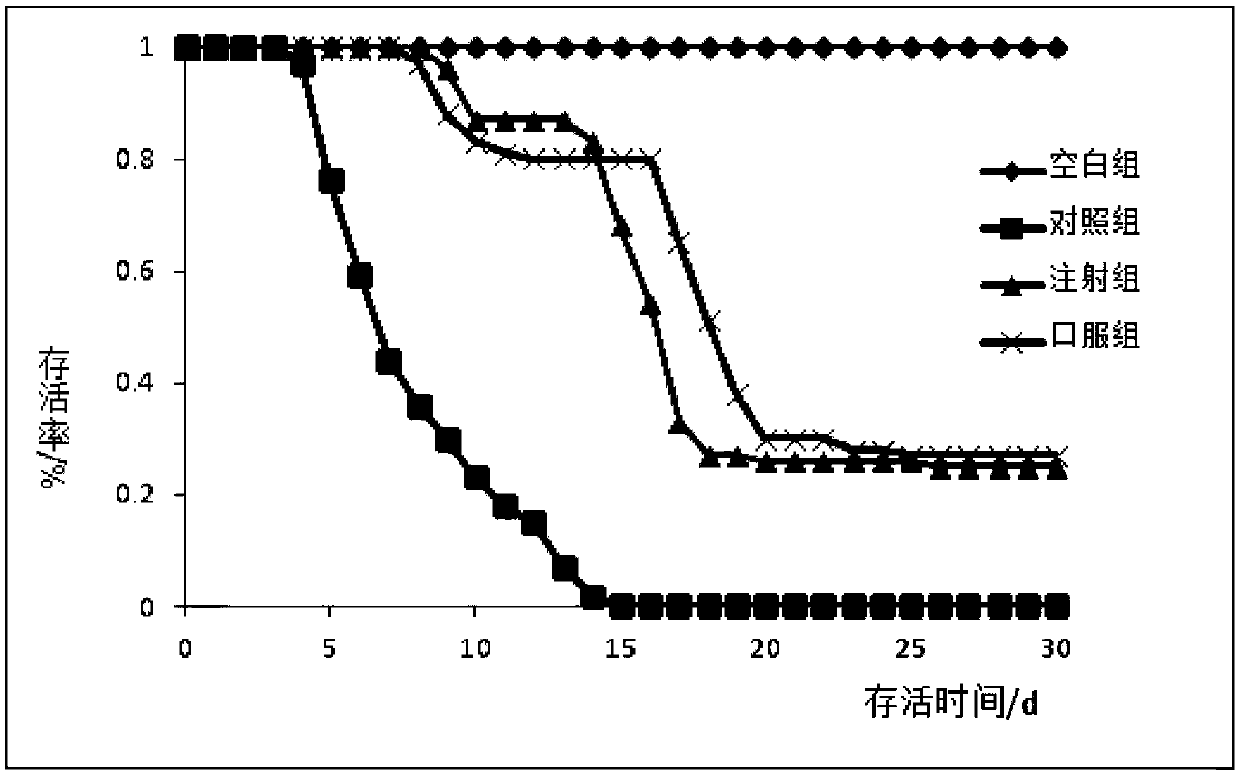
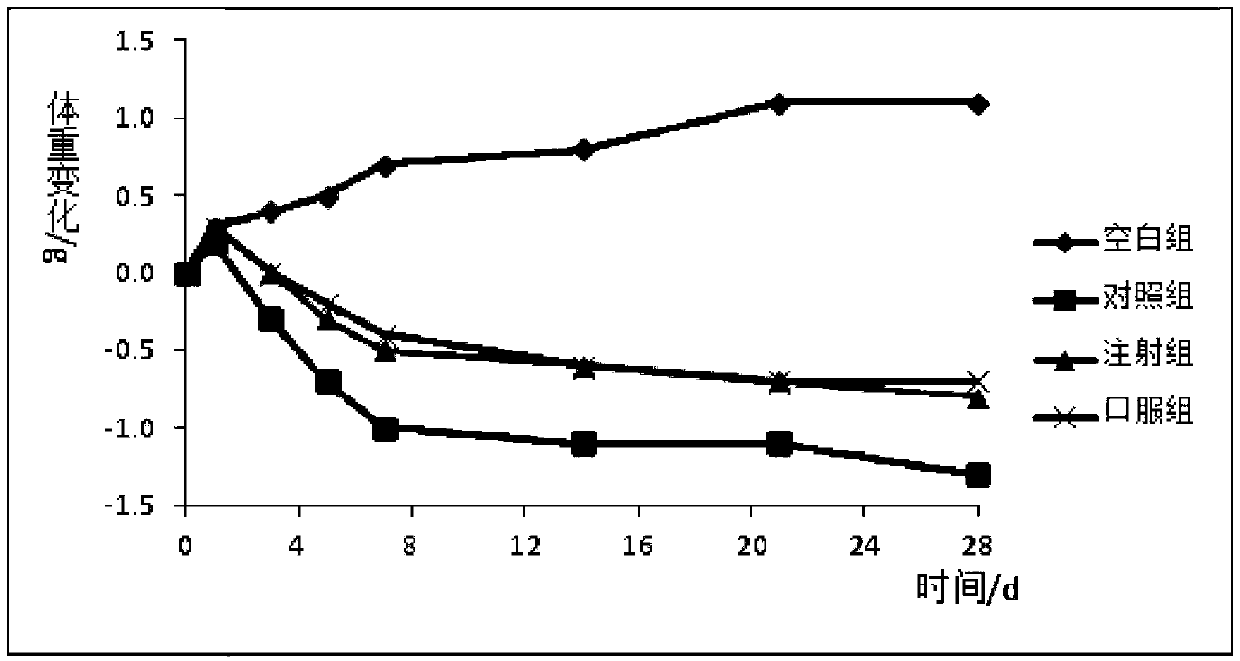
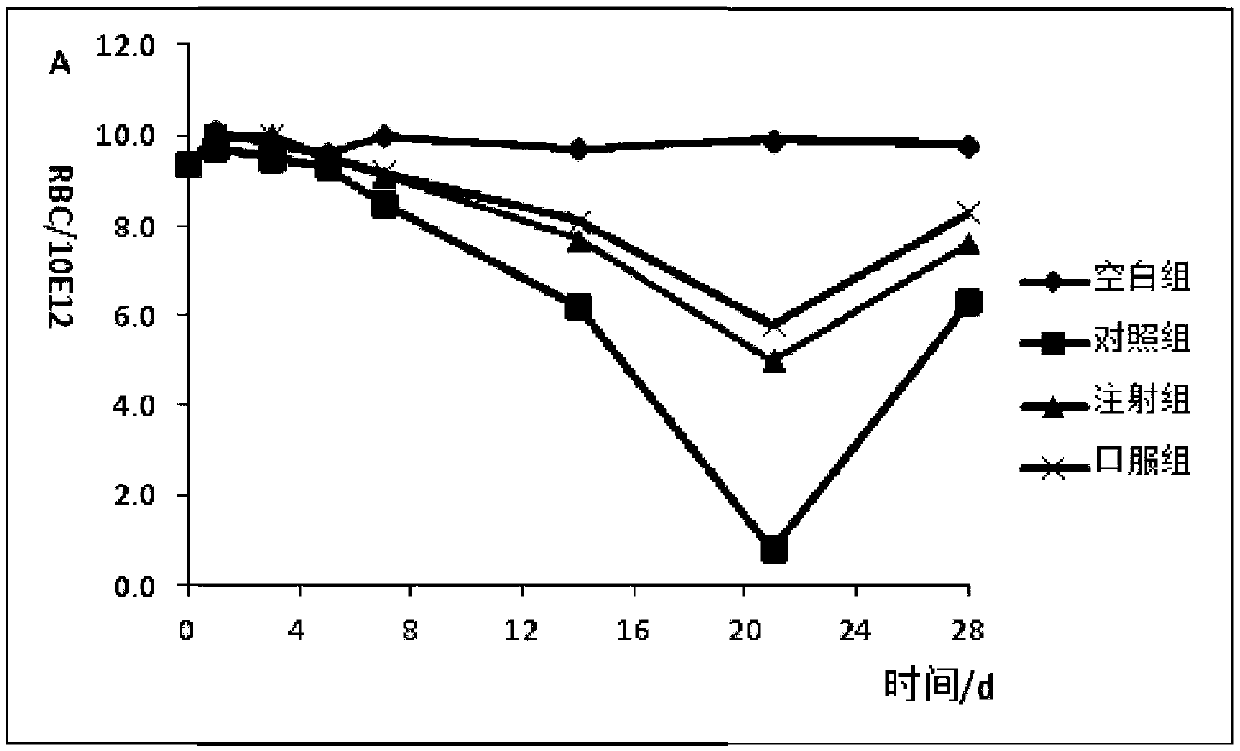
Formula and preparation technology for estriol nano oral preparation
A technology of oral preparation and preparation process, which is applied in the field of medicine, can solve the problems of low oral bioavailability, high side effects of estrogen, inconvenient use of injections, etc., achieve good prevention and treatment effects, improve curative effect, and be easy to carry and use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1 prepares estriol nanocrystal suspension with different stabilizers
[0051] prescription:
[0052]
[0053] method:
[0054] Dissolve 1.0 g of sodium lauryl sulfate and 5.0 g of stabilizer (poloxamer 188, poloxamer 407, HPMC E3, PVP K30 for the four groups respectively) in 100 ml of purified water, and add the 10.0 g of estriol bulk drug that has been air-pulverized to 2 μm is mixed uniformly, and then sheared by a high-speed shear disperser at 10,000 r / min for 5 minutes to fully suspend. Utilize the planetary high-energy ball mill (P-7, Mercedes-Benz, Germany) to grind the suspension for a suitable time, and use the American PSS particle size tester to measure the drug particle distribution results as follows:
[0055] Table 1 Investigation of the influence of different stabilizers on grinding
[0056]
[0057] The results showed that grinding with four stabilizers could effectively reduce the particle size of estriol raw material. However, Polox...
Embodiment 2
[0058] Example 2 Preparation of estriol nanocrystal suspension with different charge protecting agents
[0059] prescription:
[0060]
[0061] method:
[0062] Dissolve 1.0g of charge protection agent (three groups of sodium lauryl sulfate, sodium docusate, sodium lauryl sulfate: sodium docusate = 1:1) and 5.0 g of poloxamer 188 in sequence In 100ml of purified water, add 10.0g of estriol raw material that has been air-pulverized to 2μm under stirring state to make it evenly mixed, and then shear it through a high-speed shear dispersing homogenizer at 10000r / min for 5min to fully suspend. Utilize the planetary high-energy ball mill (P-7, Mercedes-Benz, Germany) to grind the suspension for a suitable time, and use the American PSS particle size tester to measure the drug particle distribution results as follows:
[0063] Table 2 Investigation of the Effects of Different Charge Protectants on Grinding
[0064]
[0065] The results showed that grinding with sodium laury...
Embodiment 3
[0066] Example 3 Preparation of estriol nanocrystal suspension with different concentrations of raw materials
[0067] prescription:
[0068]
[0069] method:
[0070] Dissolve 1.0 g of sodium lauryl sulfate and 5.0 g of poloxamer in 100 ml of purified water in sequence, and add 0.1 g, 0.5 g, 1 g, 3 g, and 5 g of estriol raw materials that have been jet-milled to 2 μm in advance under stirring , 8g, 10g, 12g, 15g, 20g to make it evenly mixed, and then through a high-speed shear dispersing homogenizer 10000r / min shear 5min to fully suspend. Utilize the planetary high-energy ball mill (P-7, Mercedes-Benz, Germany) to grind the suspension for a suitable time, and use the American PSS particle size tester to measure the drug particle distribution results as follows:
[0071] Table 3 API Concentration Investigation
[0072]
[0073]
[0074] The results show that after grinding, the particle size and the concentration of the raw material drug form a U-shaped distributio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com