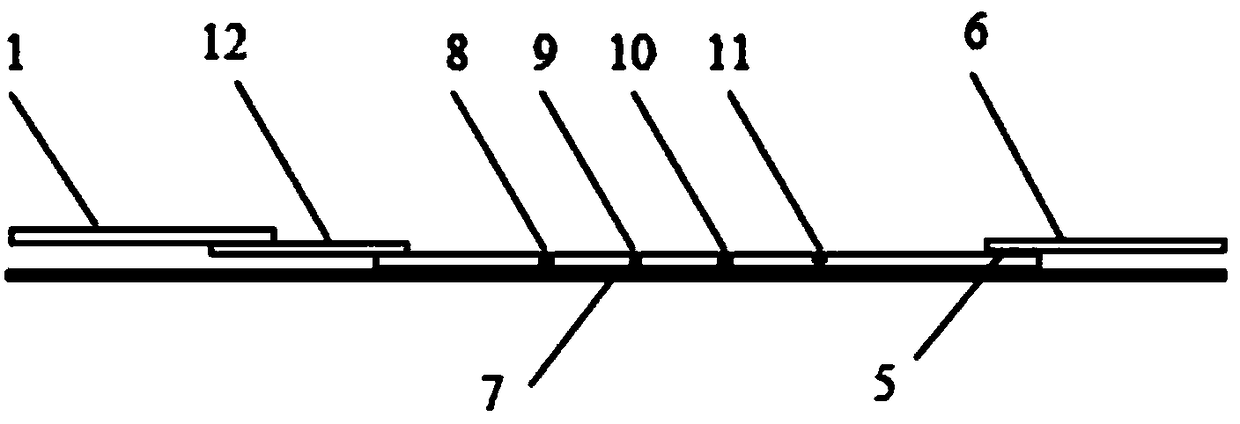
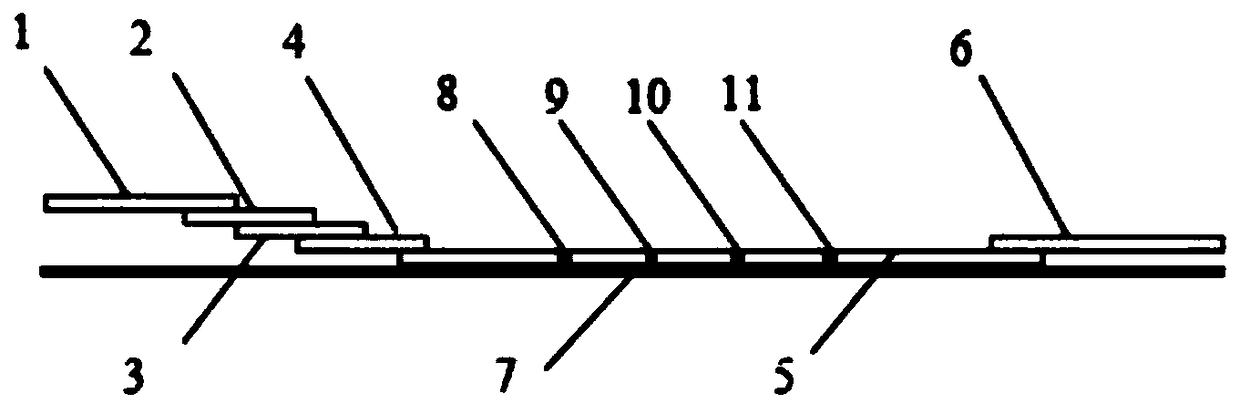
Fluorescence immunochromatography kit for simultaneously detecting CRP, lipoprotein phospholipase A2 and D-dimers and preparation method thereof
A technique of fluorescence immunochromatography and immunochromatography test strips, applied in the field of medical testing, can solve the problems of multiple samples, missed diagnosis and treatment opportunities, high testing costs and labor costs, and achieves reduction of industrial production costs, simple structure, and improved detection and efficiency. The effect of diagnostic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] A preparation method of a fluorescent immunochromatography kit for simultaneously detecting ultrasensitive CRP, lipoprotein phospholipase A2 and D-dimer, the preparation method comprising the following steps:
[0053] (a) Treatment of fluorescent substances:
[0054] Dissolve carboxyfluorescein in morpholineethanesulfonic acid (MES) buffer solution with a pH of 5.0. After carboxyfluorescein is fully dissolved, add the activator 1-(3-dimethylaminopropyl)-3-ethane Carbodiimide (EDC), mix evenly, then add the activator N-hydroxysuccinimide (NHS) equal to EDC, mix evenly, incubate for 0.5h at room temperature in the dark, and then centrifuge Centrifuge for 5 minutes, take out the supernatant, set aside, wash and redissolve the precipitate with morpholineethanesulfonic acid (MES) buffer solution with pH 5.0, the concentration of EDC and NHS is 1mg / mL;
[0055] (b) Antibody labeling:
[0056] Add the hs-CRP monoclonal antibody to the carboxyfluorescein solution obtained in ...
Embodiment 2
[0068] A preparation method of a fluorescent immunochromatography kit for simultaneously detecting ultrasensitive CRP, lipoprotein phospholipase A2 and D-dimer, the preparation method comprising the following steps:
[0069] (a) Treatment of fluorescent substances:
[0070] Dissolve carboxyfluorescein in morpholineethanesulfonic acid (MES) buffer solution with a pH of 5.0. After carboxyfluorescein is fully dissolved, add the activator 1-(3-dimethylaminopropyl)-3-ethane Carbodiimide (EDC), mix evenly, then add the activator N-hydroxysuccinimide (NHS) equal to EDC, mix evenly, incubate for 0.5h at room temperature in the dark, and then centrifuge Centrifuge for 5 minutes, take out the supernatant, set aside, wash and redissolve the precipitate with morpholineethanesulfonic acid (MES) buffer solution with pH 5.0, the concentration of EDC and NHS is 1mg / mL;
[0071] (b) Antibody labeling:
[0072]Add the hs-CRP monoclonal antibody to the carboxyfluorescein solution obtained in s...
Embodiment 3
[0083] A preparation method of a fluorescent immunochromatography kit for simultaneously detecting ultrasensitive CRP, lipoprotein phospholipase A2 and D-dimer, the preparation method comprising the following steps:
[0084] (a) Treatment of fluorescent substances:
[0085] Dissolve fluorescein cy5 in morpholineethanesulfonic acid (MES) buffer solution with a pH of 6.0. After fluorescein cy5 is fully dissolved, add the activator 1-(3-dimethylaminopropyl)-3-ethane Carbodiimide (EDC), mix evenly, then add the activator N-hydroxysuccinimide (NHS) equal to EDC, mix evenly, incubate for 1.5h at room temperature in the dark, and then centrifuge Centrifuge for 15 minutes, take out the supernatant, set aside, wash and redissolve the precipitate with morpholineethanesulfonic acid (MES) buffer solution with pH 6.0, the concentration of EDC and NHS is 1mg / mL;
[0086] (b) Antibody labeling:
[0087] Add the hs-CRP monoclonal antibody to the fluorescein cy5 solution obtained in step (a)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com