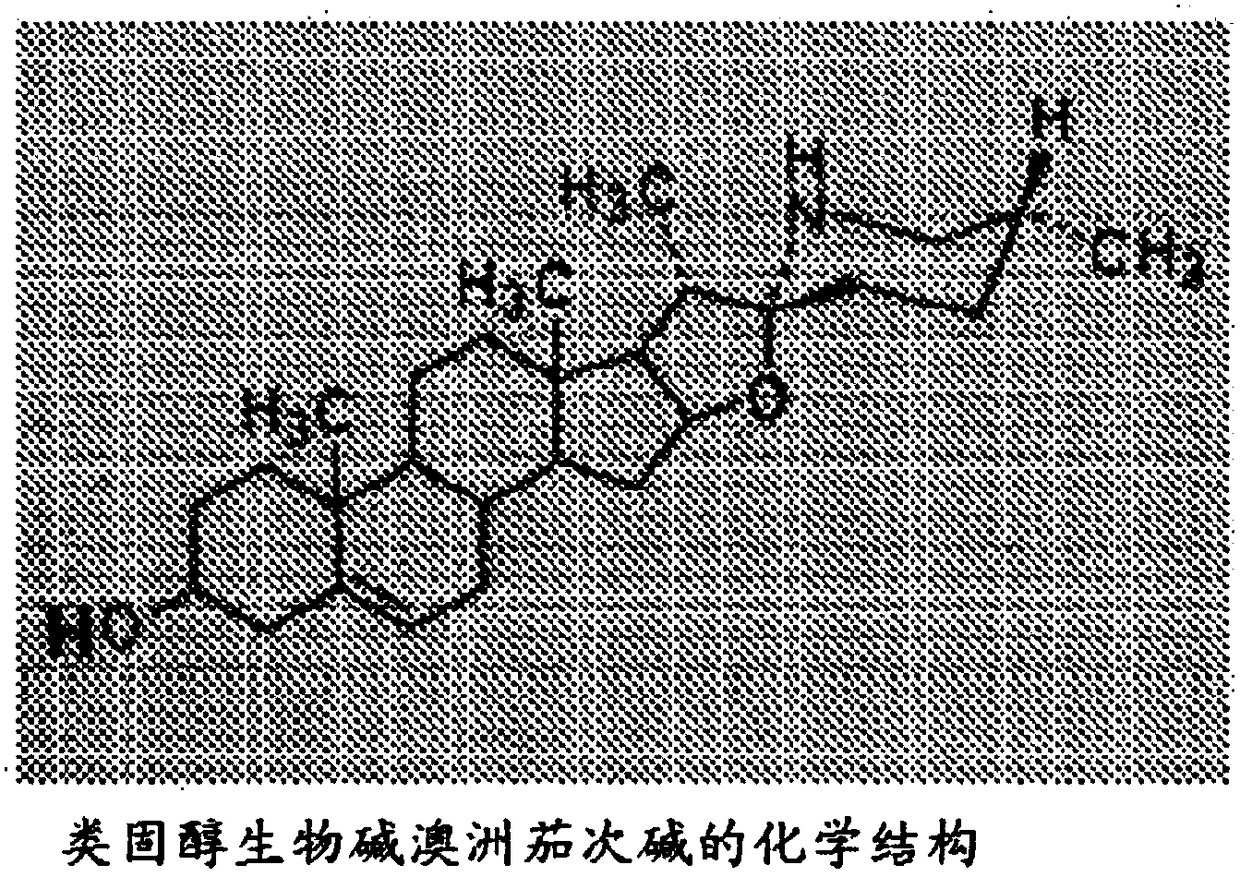
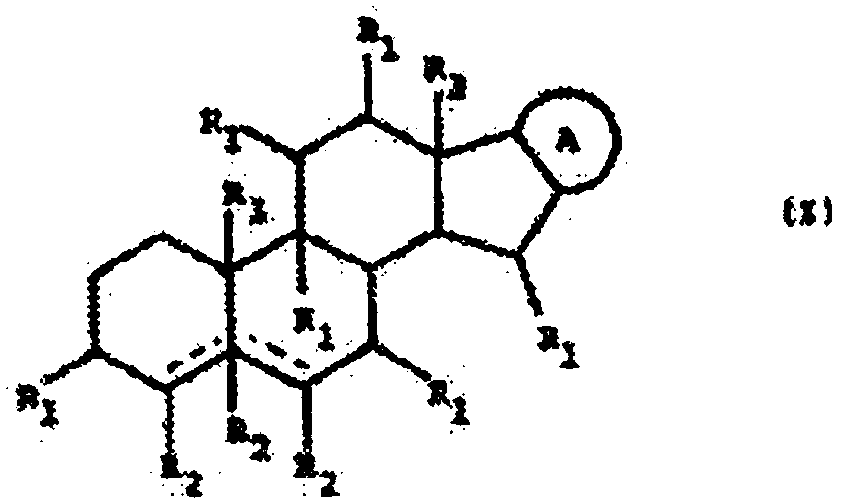
Glycoalkaloid combinations and various uses thereof
A technology of alkaloids and glycosides, applied in the field of therapeutic compositions, can solve problems such as reduced anti-tumor efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0211] Example 1: Preparation of sugar-free solanine glycosides
[0212] This example illustrates a method of preparing a glycoalkaloid formulation that is substantially free (ie, free of) free sugars, including types that inhibit the interaction between the glycoalkaloid and its target cells of free sugars.
[0213] A sugar-free solanine glycoside formulation was prepared as follows: 50 kg of Solanum Sodomaeum berries were passed through a commercial meat grinder with a mesh size of 3 mm (equipped with a 1.HP motor 1425 rpm).
[0214] The slurry was diluted to a volume of 200L with 3% acetic acid (pH 2.5) (food grade). This semisolid solution was treated with a Silverson homogenizer for 15 minutes. Mixing was continued for an additional 4 hours at room temperature at 30 rpm (FlamingoCMG 0.75kw variable speed controller) using an SS bar with an arm mixer.
[0215] The solution was left to stand overnight without mixing. The solution was then filtered through a muslin cloth...
Embodiment 2
[0219] Example 2: Stability analysis of solanine glycosides.
[0220] This example demonstrates the preparation of different topical cream formulations comprising at least one keratolytic agent and a glycoalkaloid with or without viscosity modifiers (eg, as excipients and / or carriers), and evaluates formulations Glycoalkaloid stability, and assess the stability of glycoalkaloid actives in those formulations. The presented results also demonstrate the preparation of a novel, substantially stable and effective topical composition having a long shelf life for use in therapy, the composition comprising at least one glycoalkaloid, at least one viscosity modifier and At least one keratolytic agent.
[0221] In the context of a previous human skin cancer study, a cream formulation containing glycoalkaloids was tested within five months of making the cream. The results are remarkable. From these studies, only the presence of active glycoalkaloids was shown. However, their concentr...
Embodiment 3
[0251] Example 3: Effect of free (unconjugated) sugars on the therapeutic activity of glycoalkaloids
[0252] This example demonstrates that free (unconjugated) sugar moieties such as free rhamnose reduce the therapeutic anticancer / tumor efficacy of the SR glycoalkaloids of the present invention.
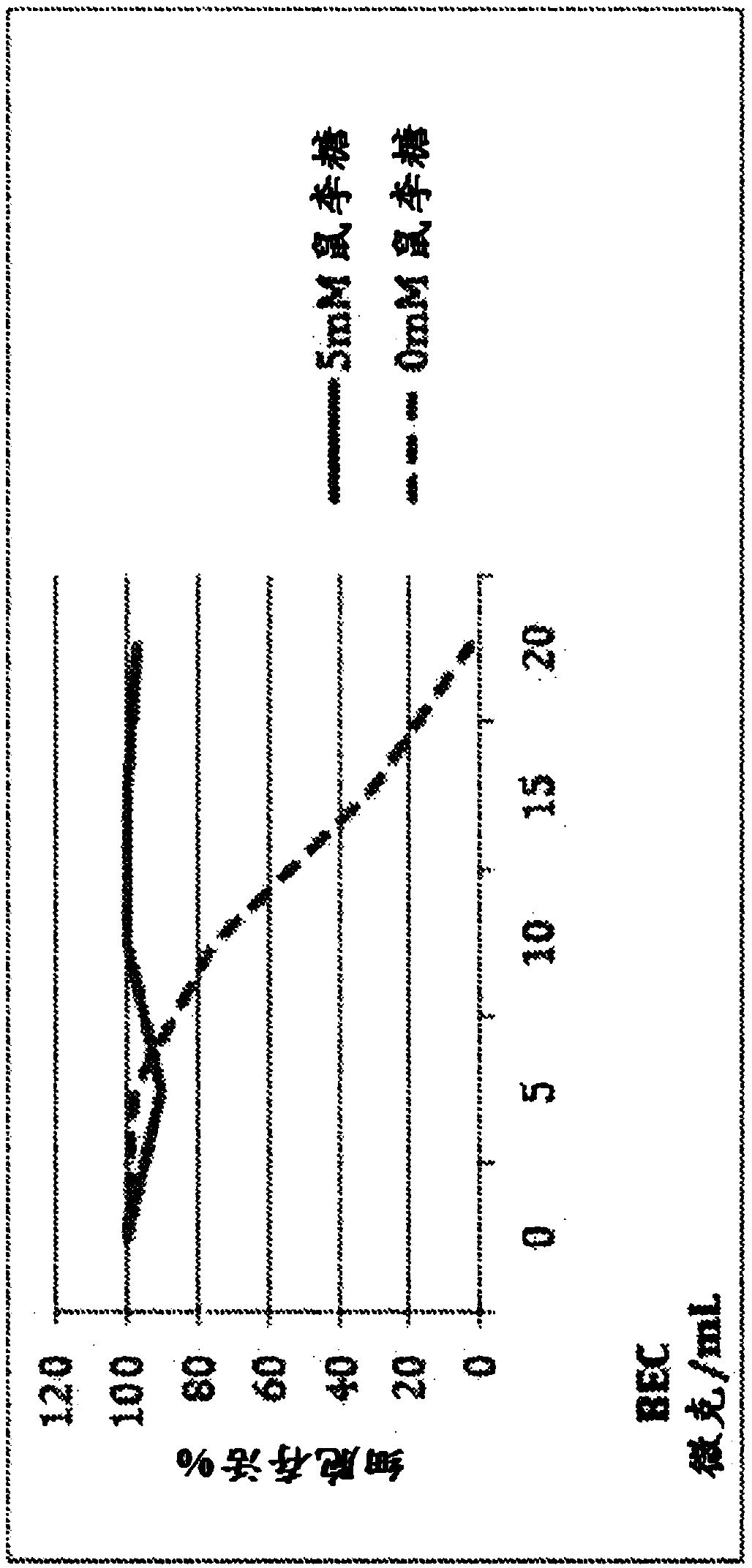
[0253] To determine the effect of the free sugar moiety on the anticancer activity of the SR glycoalkaloids of the invention, increasing concentrations of BEC glycoalkaloids were assayed under cell culture conditions in the absence of free rhamnose and in the presence of 5 mM rhamnose alone Anticancer activity of alkaloid extracts. To this end, melanoma cancer cells were treated with increasing concentrations (0-20 μg / mL) of BEC extracts (consisting of solane, solane, and solani) in the presence or absence of free rhamnose. bases consisting of a constant mixture of di- and mono-glycosides) were incubated together. The results are shown in figure 1 middle.
[0254] like figure 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com