Electrolyte injection for children
A technology for electrolyte injection and injection, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, metabolic diseases, etc. Effects of hypoglycemia or hyperglycemia, metabolic acidosis avoidance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
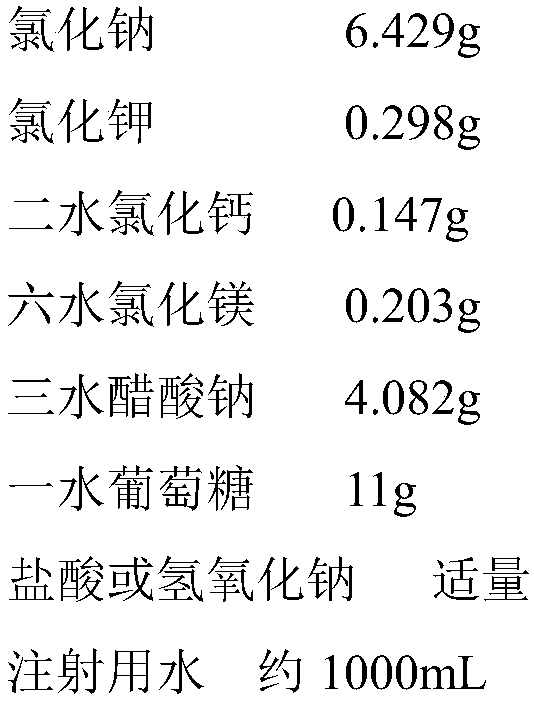
[0020] The preparation formula of electrolyte supplement injection for children is as follows:
[0021]
[0022] The preparation method is as follows
[0023] Dilute preparation: add 40-75℃, 40%-80% volume fresh water for injection into the dilute preparation tank, weigh each raw material of the prescription amount, start stirring, slowly put into the dilute preparation tank, and stir for 5-30 minutes , and then add water for injection to the prescription amount into the dilute preparation tank, continue to stir for 30 minutes, turn on the infusion pump after the dissolution is complete, and let the liquid medicine flow back through the 5um, 0.45um filter element and the return line respectively, and circulate for 10 to 30 minutes. Sampling and testing semi-finished products, after passing the test, start filling.
[0024] Filling: After filtering through 5um, 0.45um, 0.2um, microporous filter elements, adjust the filling volume to pass the filling.
[0025] Sterilization...
Embodiment 2
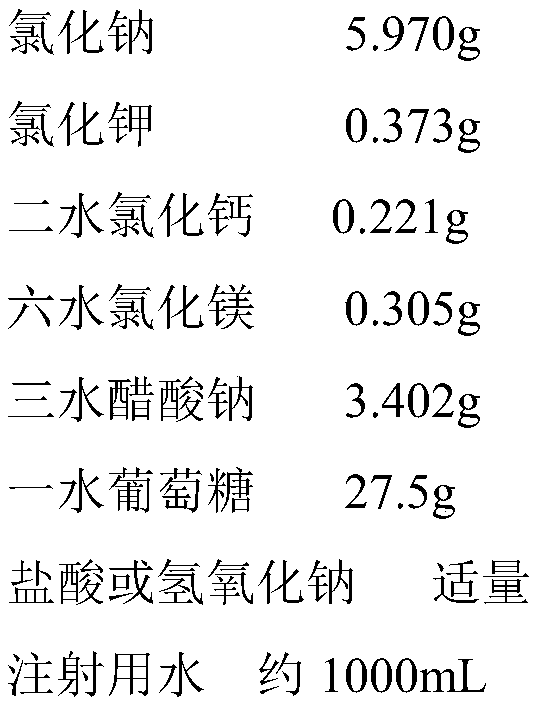
[0027] The preparation formula of electrolyte supplement injection for children is as follows:
[0028]
[0029] The preparation method is as follows
[0030] Dilute preparation: add 40-75℃, 40%-80% volume fresh water for injection into the dilute preparation tank, weigh each raw material of the prescription amount, start stirring, slowly put into the dilute preparation tank, and stir for 5-30 minutes , and then add water for injection to the prescription amount into the dilute preparation tank, continue to stir for 30 minutes, turn on the infusion pump after the dissolution is complete, and let the liquid medicine flow back through the 5um, 0.45um filter element and the return line respectively, and circulate for 10 to 30 minutes. Sampling and testing semi-finished products. After passing the test, start filling.
[0031] Filling: After filtering through 5um, 0.45um, 0.2um, microporous filter elements, adjust the filling volume to pass the filling.
[0032] Sterilizatio...
Embodiment 3
[0034] The preparation formula of electrolyte supplement injection for children is as follows:
[0035]
[0036] The preparation method is as follows
[0037] Dilute preparation: add 40-75℃, 40%-80% volume fresh water for injection into the dilute preparation tank, weigh each raw material of the prescription amount, start stirring, slowly put into the dilute preparation tank, and stir for 5-30 minutes , and then add water for injection to the prescription amount into the dilute preparation tank, continue to stir for 30 minutes, turn on the infusion pump after the dissolution is complete, and let the liquid medicine flow back through the 5um, 0.45um filter element and the return line respectively, and circulate for 10 to 30 minutes. Sampling and testing semi-finished products. After passing the test, start filling.
[0038] Filling: After filtering through 5um, 0.45um, 0.2um, microporous filter elements, adjust the filling volume to pass the filling.
[0039] Sterilizatio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Osmotic pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com