Anaerobic biodegradation method for azo dye gold orange II and treatment method for wastewater
A technology of anaerobic organisms and azo dyes, which is applied in biological water/sewage treatment, anaerobic digestion treatment, water treatment parameter control, etc., and can solve problems such as increasing the cost of biological methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] The medium composition that this embodiment adopts is:
[0047] The composition of major elements is as follows (per L): KHCO 3 ,0.5g; KH 2 PO 4 ,0.05g; MgSO 4 ·7H 2 O, 0.2g; CaCl 2 ,0.2265g; Acidic trace elements, 0.2mL; Basic trace elements, 0.5mL. The composition of acidic trace elements is as follows (per L): FeSO 4 ·7H 2 O, 2.085g; ZnSO 4 ·7H 2 O, 0.068g; CoCl 2 ·6H 2 O, 0.12g; MnCl 2 4H 2 O, 0.5g; CuSO 4 ,0.32g; NiCl 2 ·6H 2 O, 0.095g; H 3 BO 3 , 0.014g; HCl, 100mmol. The composition of alkaline trace elements is as follows (per L): Na 2 WO 4 2H 2 O, 0.05g; Na 2 MoO 4 , 0.242g; NaOH, 0.4g.
[0048] Prepare 8g / L stock solution of the azo dye Golden Orange II: weigh 2g of the azo dye Golden Orange II and dissolve it in a beaker filled with 150mL of deionized water, then stir to dissolve, and use a 250mL volumetric flask to make it to volume. Finally, transfer to a 250mL blue bottle for later use.
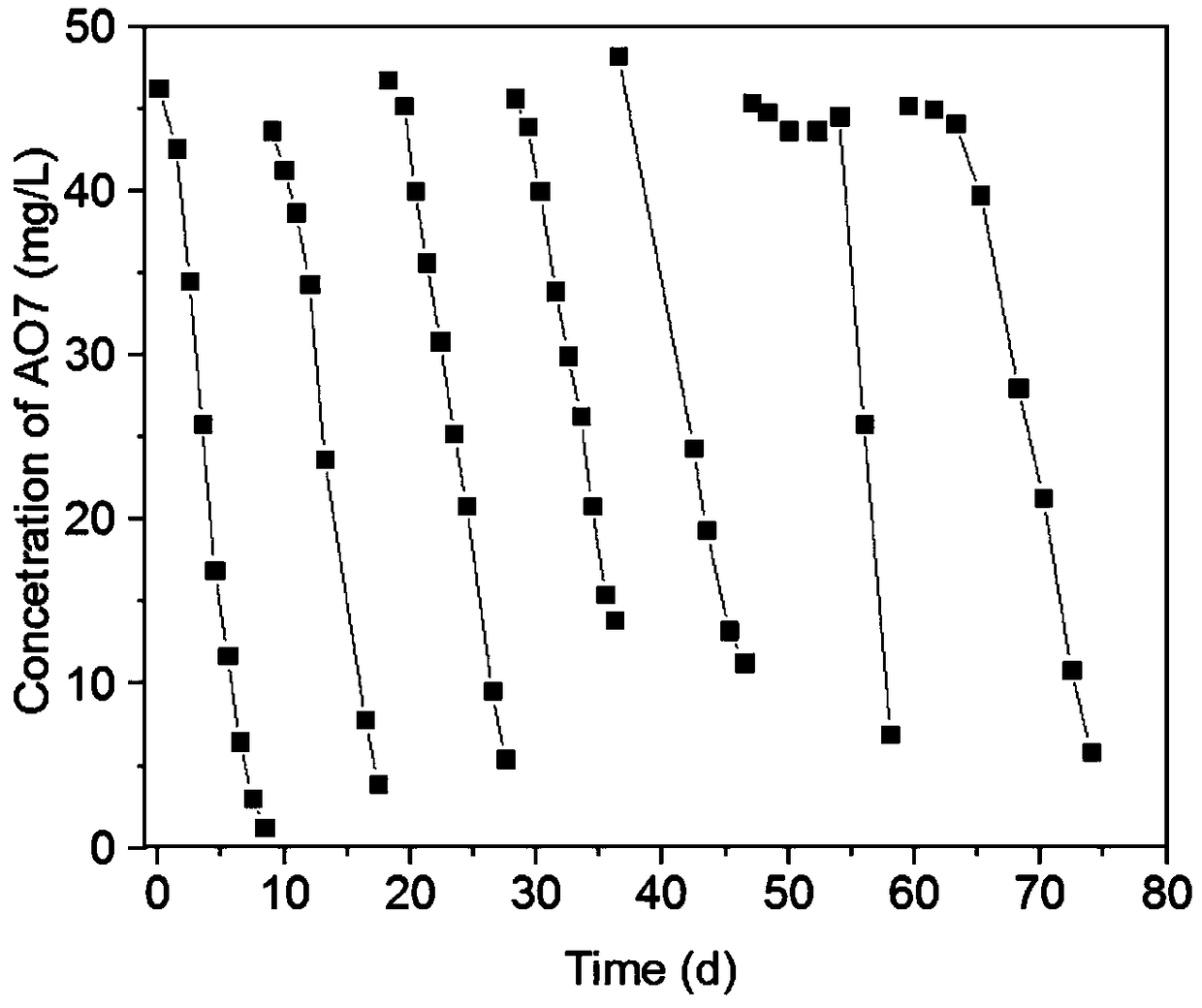
[0049] According to the following method, ...
Embodiment 2
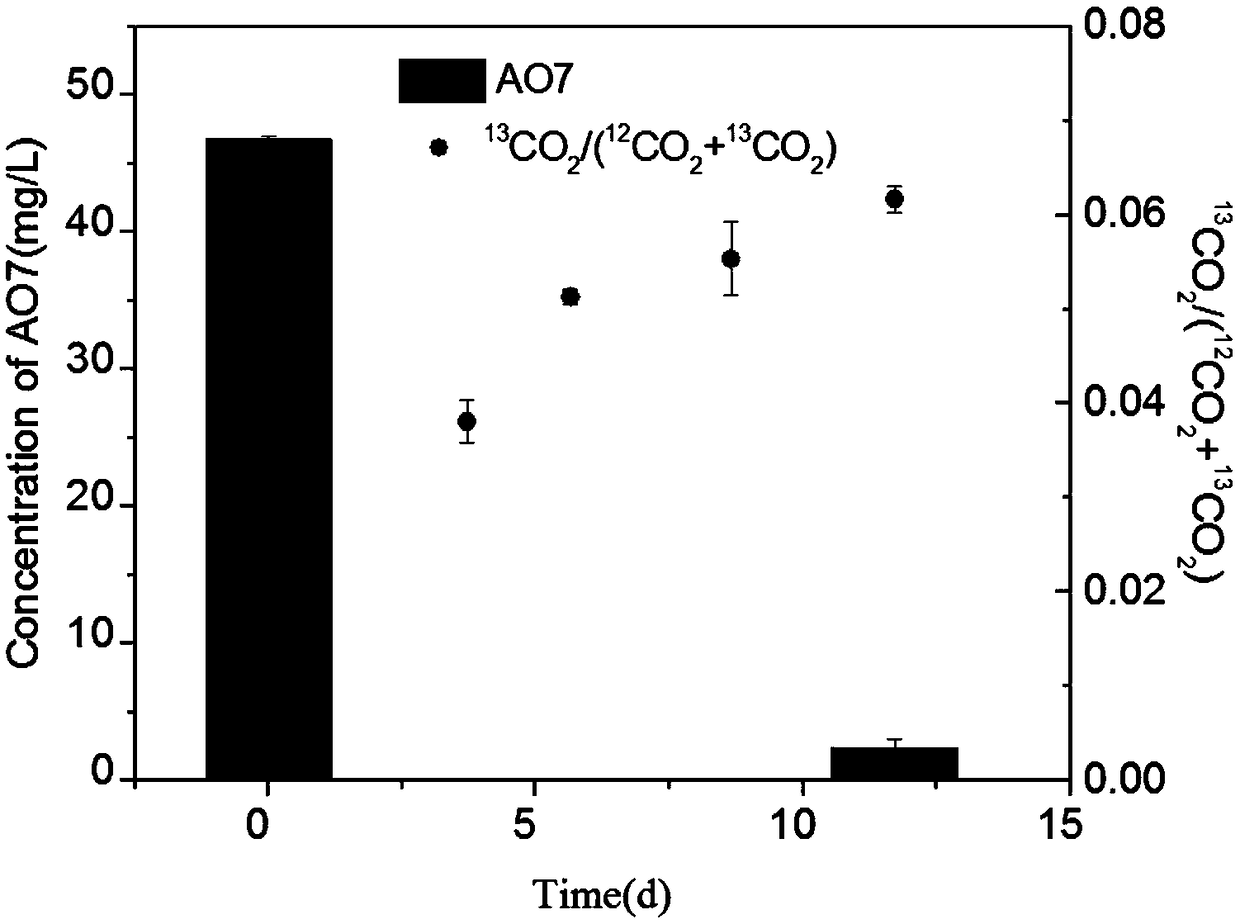
[0060] The relationship between isotope methane and concentration change of azo dye Golden Orange II
[0061] The medium composition that this embodiment adopts is:
[0062] Prepare 2g / L stock solution of azo dye Golden Orange II: Weigh 0.2g of azo dye Golden Orange II and dissolve it in a beaker filled with 50mL of deionized water, then stir to dissolve, and use a 100mL volumetric flask to volume , and finally transferred to a 100mL blue-necked bottle for later use.
[0063] Take 2 clean serum bottles with a volume of 60mL, and seal them with butyl rubber stoppers. Vacuum each for 5 minutes to ensure an anaerobic environment.
[0064] Then, take 10 mL of the inoculum slurry containing denitrifying anaerobic methane oxidizing archaea and put it into the glove box, dissolve it with 90 mL of fresh anaerobic medium and dilute it in a 150 mL beaker, mix well under a magnetic stirrer, and then transfer them separately with a 50 mL syringe Put 30mL into 2 vials (to ensure the sam...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com