Preparation method for activated-carbon-supported ruthenium-based ammonia synthesis catalyst
A technology of activated carbon and catalyst, which is applied in the field of preparation of activated carbon-supported ruthenium-based ammonia synthesis catalysts, which can solve the problems of reducing the ammonia synthesis performance of catalysts, affecting the dispersion of ruthenium metal and additives, and the decrease of dispersion, so as to achieve high ammonia synthesis reaction activity and Effect of heat resistance, good industrial application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
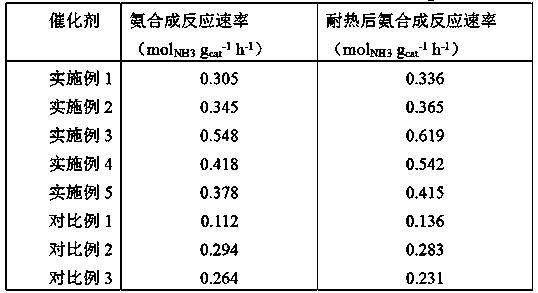
[0015] Take 5 g of activated carbon graphitized at 1850 °C in nitrogen, impregnate an equal volume of 0.05 g / mL ruthenium chloride solution to a mass ratio of Ru:C of 0.10:1, and dry at 120 °C for 4 hours; Reduction at 450°C for 6 hours, cooled to room temperature in the above atmosphere; the obtained sample was impregnated with barium nitrate aqueous solution until the molar ratio of Ba to Ru was 0.6:1, and dried at 120°C for 4 hours. Put the above sample in a tube furnace, pass through a mixed gas composed of nitric acid gas and oxygen-free gas at 300mL / min, and heat-treat at 80°C for 24 hours. In terms of volume percentage, the mixed gas is composed of: 3% nitric acid gas and 97% nitrogen gas.
Embodiment 2
[0017] Take 5g of activated carbon graphitized at 1850°C in nitrogen, impregnate an equal volume of 0.05g / mL ruthenium chloride solution to a mass ratio of Ru:C of 0.20:1, and dry at 120°C for 4 hours; Reduction at 450°C for 6 hours, cooled to room temperature in the above-mentioned atmosphere; the obtained sample was immersed in an aqueous potassium nitrate solution until the molar ratio of K to Ru was 1:1, and dried at 120°C for 4 hours. Put the above sample in a tube furnace, pass through 30mL / min of mixed gas composed of nitric acid gas and oxygen-free gas, and heat treat at 250°C for 1 hour. In terms of volume percentage, the mixed gas is composed of 90% nitric acid gas and 10% argon gas.
Embodiment 3
[0019] Take 5 g of activated carbon graphitized at 1850 °C in nitrogen, impregnate an equal volume of 0.015 g / mL nitrosoruthenium nitrate solution to a mass ratio of Ru:C of 0.3:1, and dry at 120 °C for 4 hours; Reduction at 450°C for 6 hours, and cooled to room temperature in the above atmosphere; the obtained samples were respectively impregnated with barium nitrate and potassium nitrate aqueous solutions until the molar ratios of Ba and K to Ru were 0.6:1 and 0.9:1 respectively, at 120 °C for 4 hours. Put the above sample in a tube furnace, pass through 900mL / min of mixed gas composed of nitric acid gas and oxygen-free gas, and heat treat at 150°C for 3 hours. In terms of volume percentage, the mixed gas is composed of: 20% nitric acid gas, 30% argon gas, and 50% nitrogen gas.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com