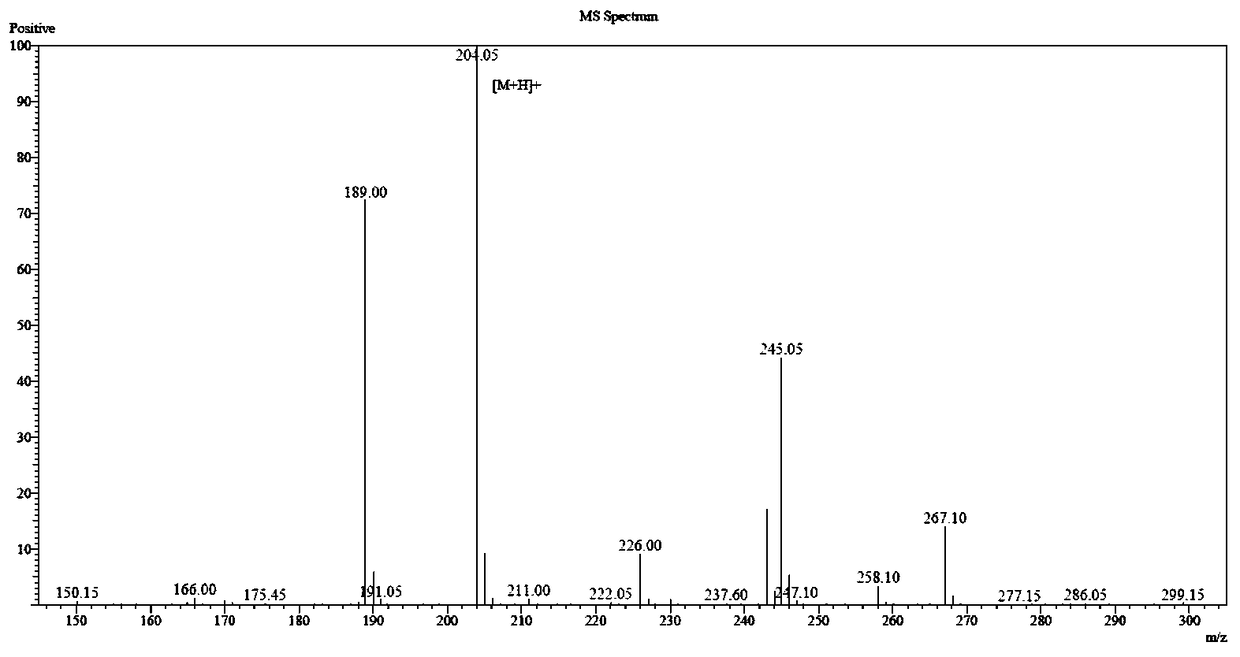
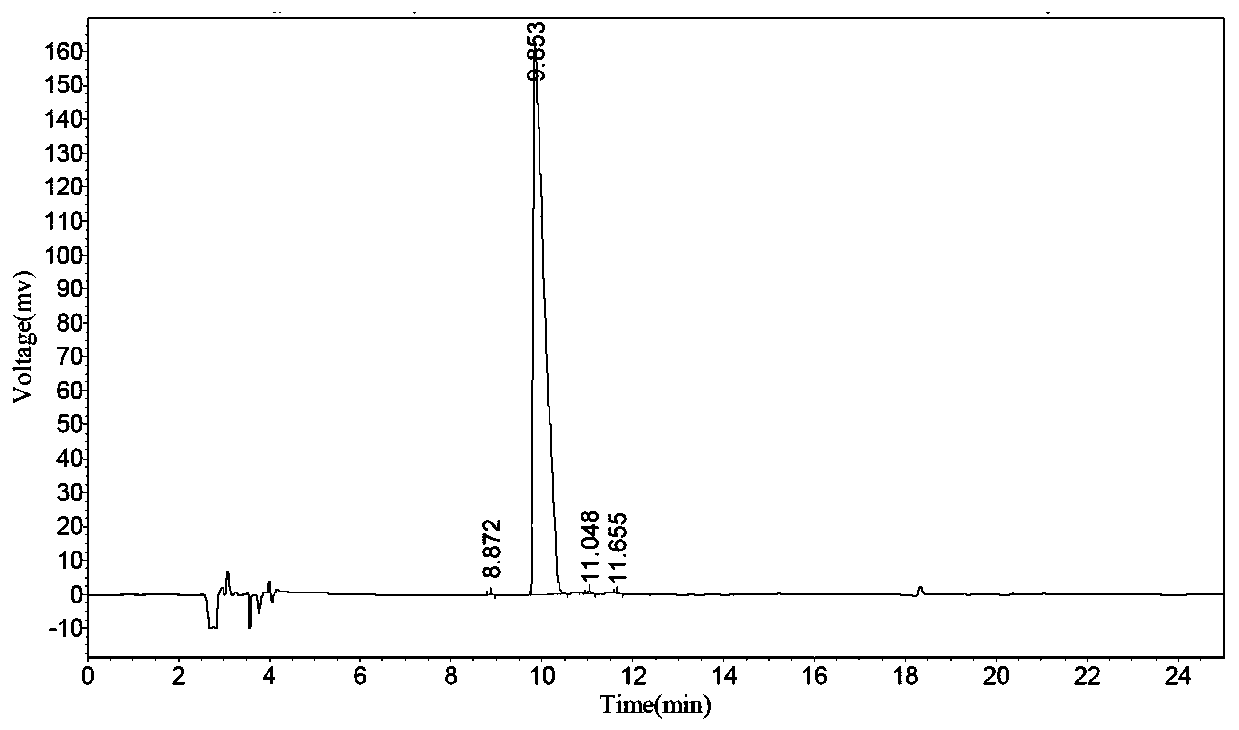
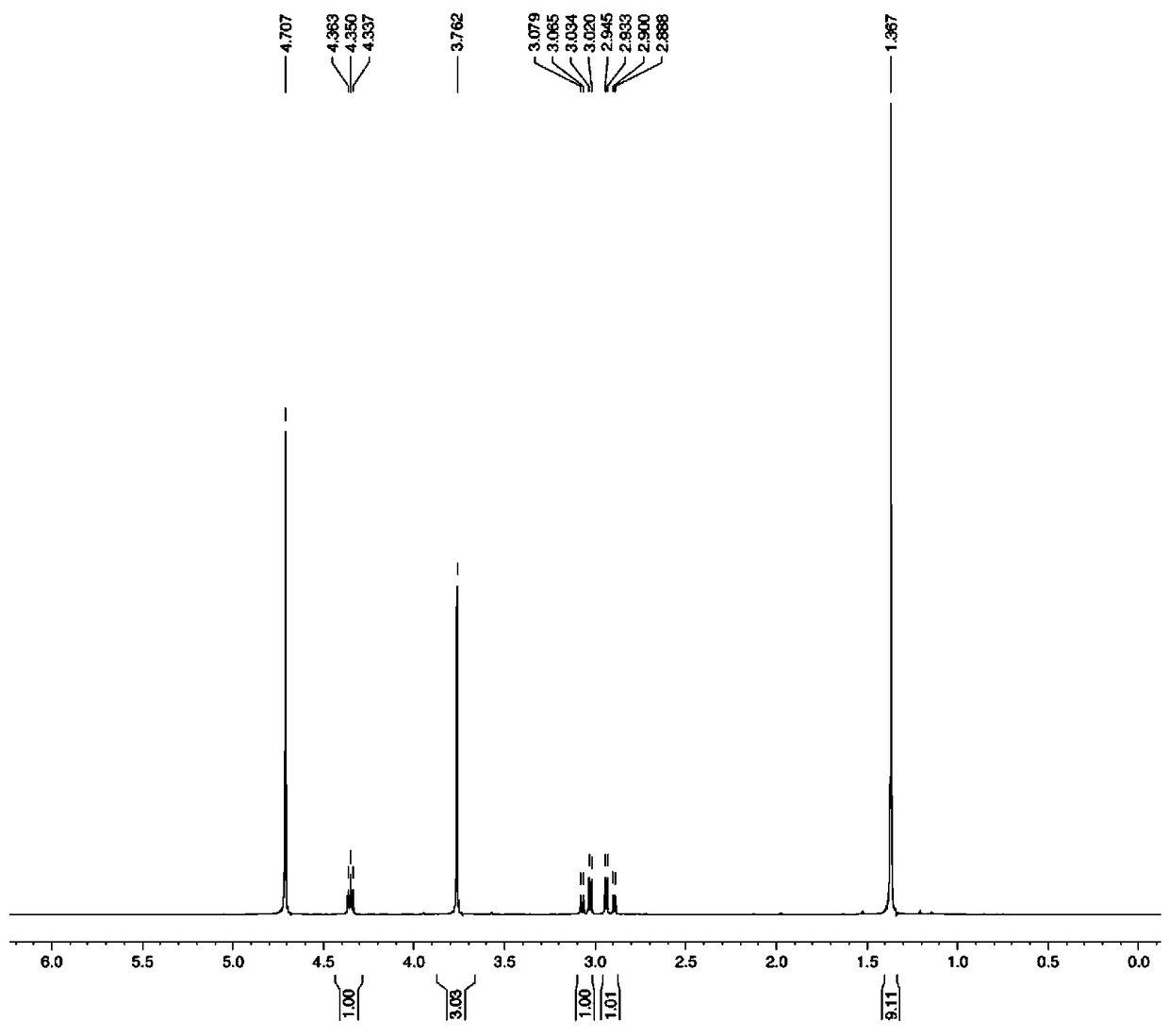
Preparation method of polypeptide raw material aspartic acid beta-tert-butyl ester alpha-methyl ester hydrochloride
A technology of methyl ester hydrochloride and aspartic acid is applied in the preparation of organic compounds, chemical instruments and methods, preparation of cyanide reactions, etc., and can solve the problems of high toxicity of methyl iodide, low yield, long cycle and the like, Achieve the effect of shortening synthesis steps, reducing production cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] In the first step, 100KG of raw material aspartic acid is suspended in 400KG of dry tetrahydrofuran, and 60KG of phosphorus oxychloride is added dropwise when the system is 10°C, and the temperature is kept below 15°C throughout the process, and the dropwise addition is completed in about 1 hour. Natural heating reaction, the reaction temperature is controlled between 15-20 ° C, and the reaction time is 12 hours. Centrifuge to obtain aspartic acid internal acid anhydride hydrochloride with a wet weight of 200KG.
[0021] In the second step, 200KG wet weight of aspartic acid anhydride hydrochloride was suspended in 800KG methanol, and reacted at 20°C for 10 hours to obtain aspartic acid α-methyl ester hydrochloride, the system was clear, and the reaction was detected by TLC. , use 70KG triethylamine to slowly adjust the pH value between 7-8, and keep the temperature not higher than 30°C to precipitate the α-methyl aspartic acid ester. After centrifugation and drying, 77...
Embodiment 2
[0026] In the first step, 100 g of raw material aspartic acid is suspended in 400 g of dry tetrahydrofuran, and 60 g of phosphorus oxychloride is added dropwise when the system is at 5°C and the temperature is kept below 15°C throughout the process, and the dropwise addition is completed in about 20 minutes. Natural heating reaction, the reaction temperature is controlled between 20-25 ° C, and the reaction time is 10 hours. Centrifuge to obtain the wet weight of aspartic acid anhydride hydrochloride 195g.
[0027] In the second step, 195g wet weight of aspartic acid anhydride hydrochloride was suspended in 800g methanol, and reacted at 20°C for 10 hours to obtain aspartic acid α-methyl ester hydrochloride, the system was clear, and the reaction was detected by TLC. , use 70g of triethylamine to slowly adjust the pH value between 7-8, and keep the temperature not higher than 30°C, so that aspartic acid α-methyl ester is precipitated. Centrifuge and dry to obtain 78.0 g of asp...
Embodiment 3
[0031] In the first step, 25KG of raw material aspartic acid is suspended in 100KG of dry tetrahydrofuran, and 15KG of phosphorus oxychloride is added dropwise when the system is at 8°C and the temperature is kept below 15°C throughout the process, and the dropwise addition is completed in about 40 minutes. The temperature is naturally raised to react, the temperature is controlled between 18-22° C. during the reaction, and the reaction time is 14 hours. Centrifuge to obtain the wet weight of aspartic acid anhydride hydrochloride 50.5KG.
[0032]In the second step, 50.5KG wet weight of aspartic acid anhydride hydrochloride was suspended in 220KG methanol, and reacted at 20°C for 10 hours to obtain aspartic acid α-methyl ester hydrochloride, the system was clear, and the reaction was detected by TLC At the end, slowly adjust the pH value between 7-8 with 17.5KG triethylamine, and keep the temperature not higher than 30°C to precipitate the α-methyl aspartic acid ester. Centrif...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com