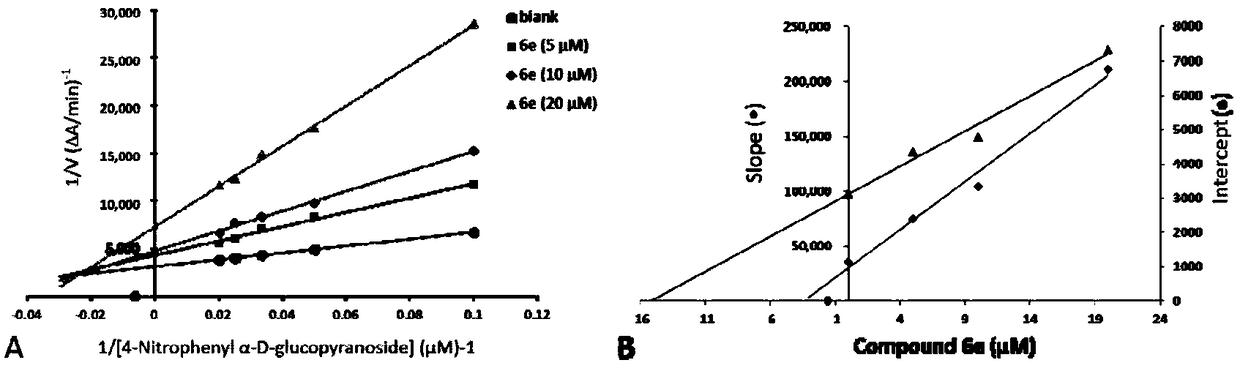
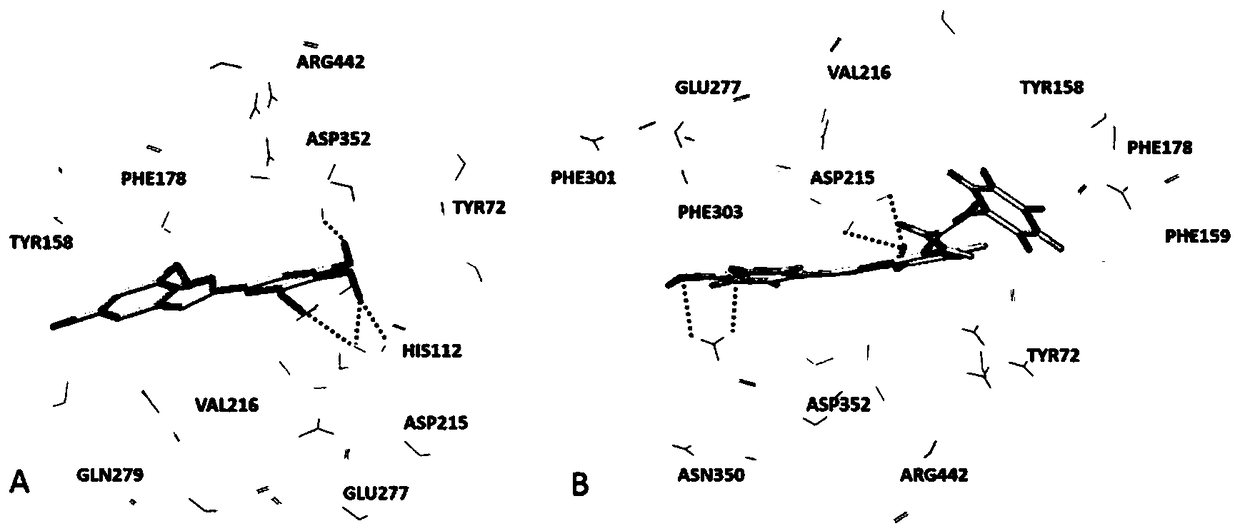
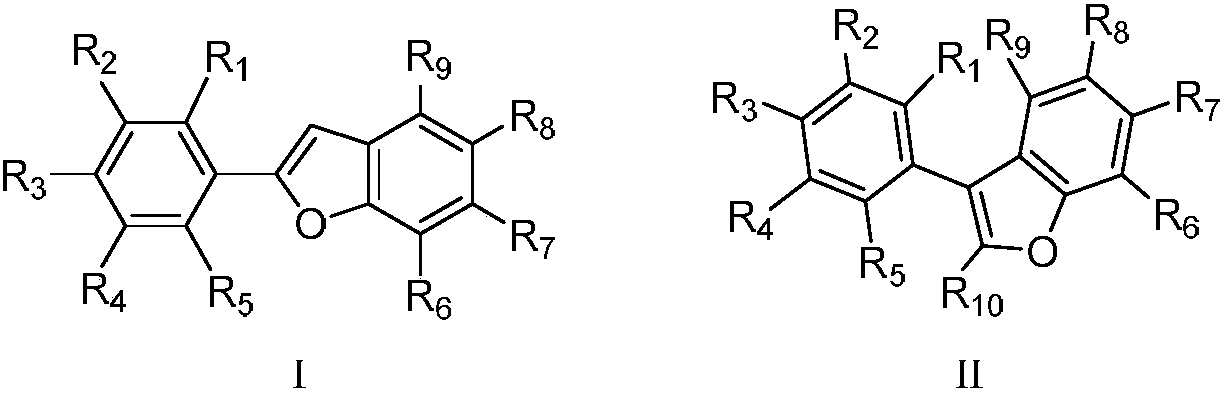
Arylbenzofuran derivative with alpha-glucosidase inhibitory activity
A technology of derivatives and arylbenzene, applied in the field of arylbenzofuran derivatives and their preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Embodiment 1 Synthesis of arylbenzofuran derivatives 6a
[0083] The general synthetic steps of target compound 6a-6g in the embodiment are as follows:
[0084]
[0085] (a) Accurately weigh an appropriate amount of 3,4-dimethoxybenzaldehyde (1) in a 25mL round bottom flask, add 4mL CBr 4 , sonicate until the sample is completely dissolved, add triphenylphosphine (2eq), and stir for 5h. The reaction was continuously monitored by TLC until the starting material spots basically disappeared. After the reaction was stopped, the solvent was evaporated to dryness. Extract with ethyl acetate (15mL×4), combine the organic layers, concentrate under reduced pressure, evaporate the solvent to dryness, wash with saturated brine, MgSO 4 dry. The sample mixture was separated and purified by silica gel column (cyclohexane / ethyl acetate 10:1→2:1 v / v) to obtain reaction intermediate 2.
[0086] (b) Accurately weigh an appropriate amount of reaction intermediate 2 in a 25mL round...
Embodiment 2
[0095] Example 2 Synthesis of arylbenzofuran derivatives 6b
[0096] The synthesis steps of the target compound 6b refer to the general synthesis steps in Example 1;
[0097] (6b)4-(4-chloro-7-hydroxybenzofuran-2-yl)benzene-1,2-diol
[0098] The structures of the reaction products obtained in the above steps were analyzed.
[0099] Physical and chemical properties: Yield: 62%, dark yellow powder (TLC R f =0.53 chloroform / methanol 4:1). Spectrum information: 1HNMR (CD 3 OD,300MHz)δ7.40(s,1H),7.28(s,1H),7.13(d,J=8.4Hz,1H),7.05(d,J=8.1Hz,1H),6.92(d,J= 8.1Hz, 1H), 6.71(d, J=8.4Hz, 1H).13C NMR (CD 3 OD,75MHz)δ153.6,148.7,146.1,144.1,143.8,131.7, 128.5,123.4,123.1,119.2,111.7,110.8,107.4,103.4.HR-MS(ESI)m / z:found 275.0114[M-H]-,calcd .for C 14 h 8 ClO 4 275.0111.
[0100] According to the above high-resolution mass spectrometry and nuclear magnetic spectrum information, the chemical formula of compound 6b can be identified as C 14 h 9 ClO 4 , its structure is as follow...
Embodiment 3
[0102] Example 3 Synthesis of arylbenzofuran derivatives 6c
[0103] The synthesis steps of the target compound 6c refer to the general synthesis steps in Example 1;
[0104] (6c)5-(4-bromo-7-hydroxybenzofuran-2-yl)benzene-1,2,3-triol
[0105] The structures of the reaction products obtained in the above steps were analyzed.
[0106] Physical and chemical properties: Yield: 71%, dark yellow powder (TLC R f =0.54 chloroform / methanol 4:1). Spectrum information: 1HNMR (CD 3 OD,300MHz)δ7.44(s,1H),7.19(s,1H),7.15(d,J=8.4Hz,1H),7.04(s,1H),6.84(d,J=8.4Hz,1H) .13C NMR (CD 3 OD,75MHz)δ 155.6,146.5,143.4,142.9,141.3,134.7,128.1,123.7,120.6,115.2,111.8,110.2, 108.0,105.4.HR-MS(ESI)m / z:found 334.9558[M-H]-, calcd.for C 14 h 8 BrO 5 334.9555.
[0107] According to the above high-resolution mass spectrometry and nuclear magnetic spectrum information, the chemical formula of compound 6c can be identified as C 14 h 9 BrO 5 , its structure is as follows:
[0108]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com