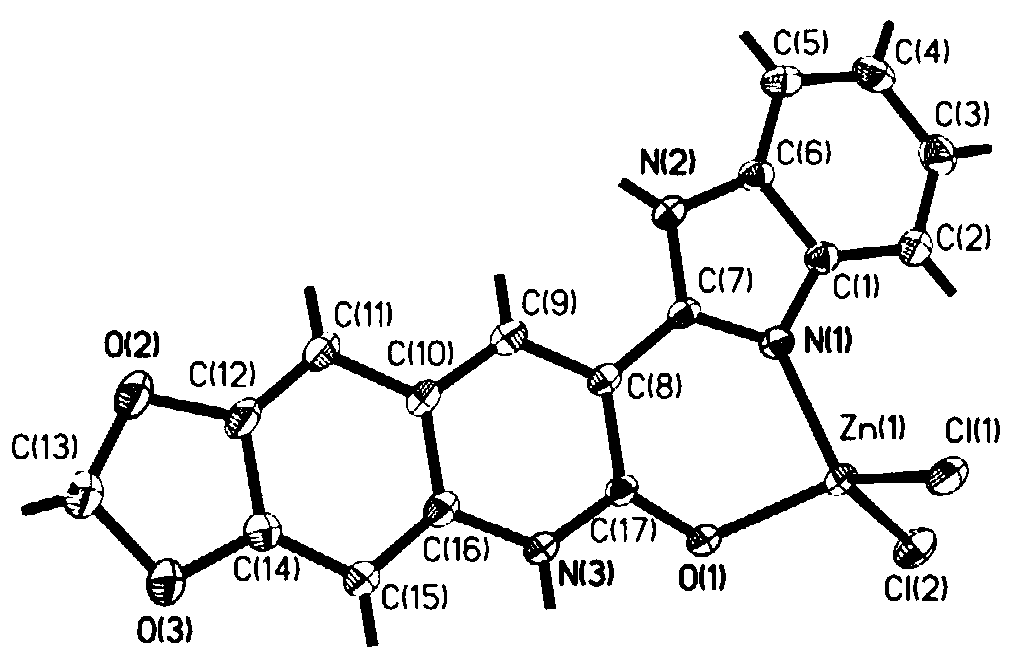
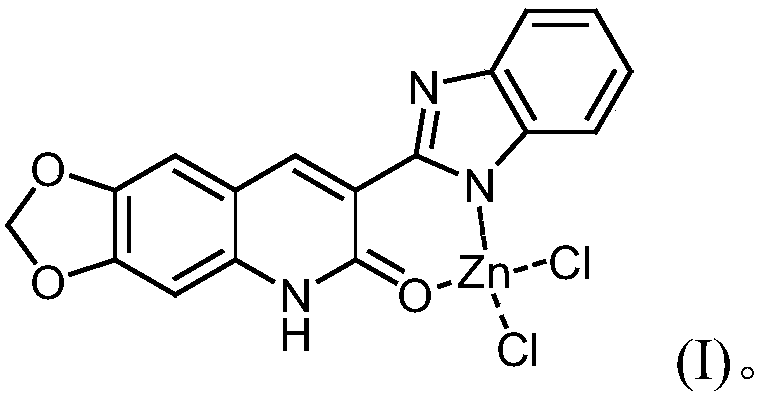
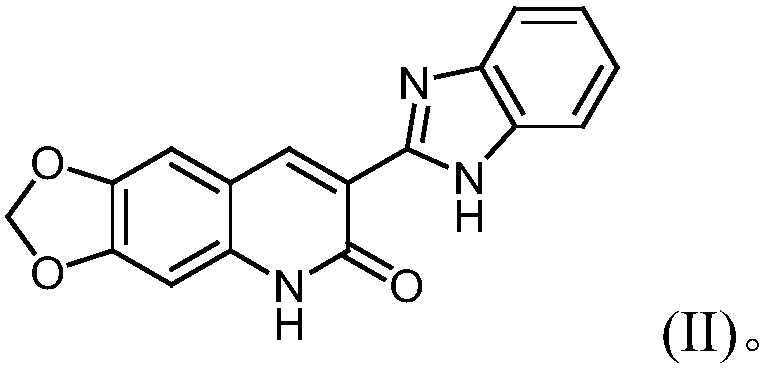
3-benzimidazole-6, 7-piperonylcyclo-2(1H)-quinolinone-zinc complex and preparation method and application thereof
A compound and reaction technology, applied in the field of medicine, can solve the problems of drug resistance, no 3-benzimidazole, and large toxic and side effects, and achieve the effects of stable quality, short preparation cycle and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Put 3.05g of compound (10mmol) shown in formula (II) and 1.40g of zinc chloride (11mmol), 10mL of methylene chloride and 10mL of methanol into the autoclave, then add 1mL of triethylamine, stir well (at this time the system pH = 7.0), the temperature was raised to 90°C for 14 hours. After the reaction, it was cooled, and yellow-green crystals were precipitated. The crystals were collected and dried to obtain 2.69 g of yellow-green crystals, with a yield of 60.50%.
[0025] The product obtained in this implementation is characterized by infrared, proton nuclear magnetic resonance spectrum, carbon spectrum, mass spectrum and X-single crystal diffraction, as follows:
[0026] (1) IR(KBr): 3432(m, NH), 2967, 2923(w, Ar-H), 2335(w, C=C), 1645(m, C=O), 1632(m, Ar- H),1048,1037(m,C-O-C)cm –1 ;
[0027] (2) 1 H NMR (500MHz, DMSO-d 6 )δ: 12.90(s,brd,1H,NH),12.65(s,brd,1H,NH),8.97(s,1H,H-Ar),7.72(s,2H,H-Ar),7.44(s ,1H,H-Ar),7.25(s,2H,H-Ar),6.88(s,1H,H-Ar),6.18(s,2H,OCH 2 O)...
Embodiment 2
[0038] Take 3-benzimidazole-6,7-pipercycline-2(1H)-quinolinone (305mg, 1mmol), 140mg of zinc chloride (1.1mmol), 8mL of dichloromethane and 8mL of methanol into an autoclave , then added 0.1 mL of triethylamine, stirred evenly (pH of the system at this time = 5.8), raised the temperature to 85° C. and reacted for 16 h, and obtained 200 mg of yellow-green crystals after cooling. Yield 45.00%.
[0039] The product obtained in this example was analyzed by infrared, hydrogen nuclear magnetic resonance, carbon spectrum, mass spectrum and X-single crystal diffraction, and it was determined that the product obtained in this example was the target compound.
Embodiment 3
[0040] Example 3: Preparation of 3-benzimidazole-6,7-piperone-2(1H)-quinolinone-zinc complex
[0041] Take 3-benzimidazole-6,7-pipercycline-2(1H)-quinolinone (610mg, 2mmol), 285mg of zinc chloride (1.2mmol), 10mL of dichloromethane and 5mL of ethanol into an autoclave , then add 5 drops of triethylamine (you can’t use ammonia water, other products will be obtained), stir evenly (the pH of the system at this time=5.0), heat up to 65°C and react for 16h, and after cooling, 492.25 mg of yellow-green crystals are obtained. Yield 55.00%.
[0042] The product obtained in this example was analyzed by infrared, hydrogen nuclear magnetic resonance, carbon spectrum, mass spectrum and X-single crystal diffraction, and it was determined that the product obtained in this example was the target compound.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com