Fluorescent probe for detecting hydrogen peroxide, and synthesis method and application thereof
A fluorescent probe and hydrogen peroxide technology, applied in the field of applied biology, can solve the problems of long synthesis time of fluorescent probes, failure to eliminate the influence of pseudo-Stokes shift, complex preparation, etc., achieve short synthesis time and avoid self-absorption and self-quenching, good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] About the synthetic method of fluorescent probe, comprise following experimental steps:
[0038] (1) Compound I (329 mg, 1 mmol), Compound II (437 mg, 1 mmol), dicyclohexylcarbodiimide (206 mg, 1 mmol) and 4-dimethylaminopyridine (12 mg, 0.1 mmol) were dissolved in 10 mL of dichloro In methane, the reaction was stirred at room temperature for 12 hours;
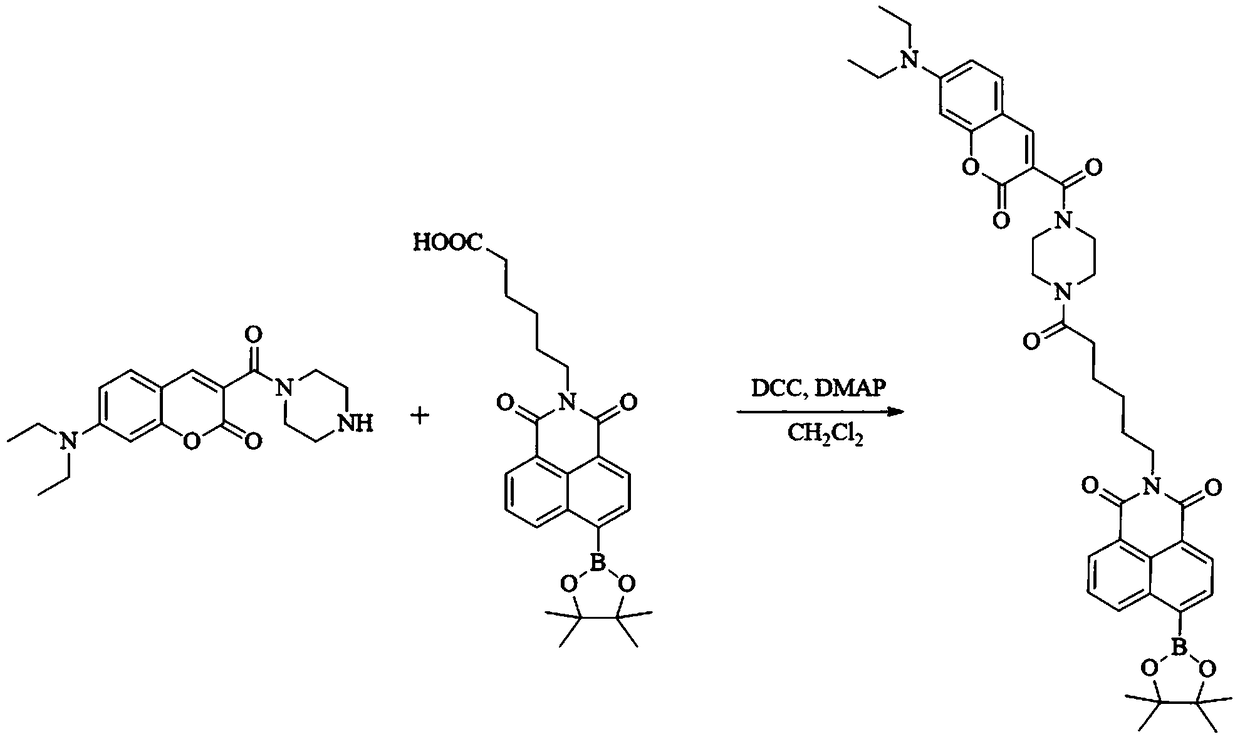
[0039] Wherein the structural formula of compound I is as follows:
[0040]
[0041] The structural formula of compound II is as follows:
[0042]
[0043] (2) After the reaction was completed, the crude product was obtained by evaporation under reduced pressure, and the crude product was treated with silica gel column chromatography (ethyl acetate:petroleum ether=1:2, V:V) to obtain a white product (628mg, yield 84% ), the white product—that is, the synthetic reaction equation of the fluorescent probe is as follows figure 1 shown.
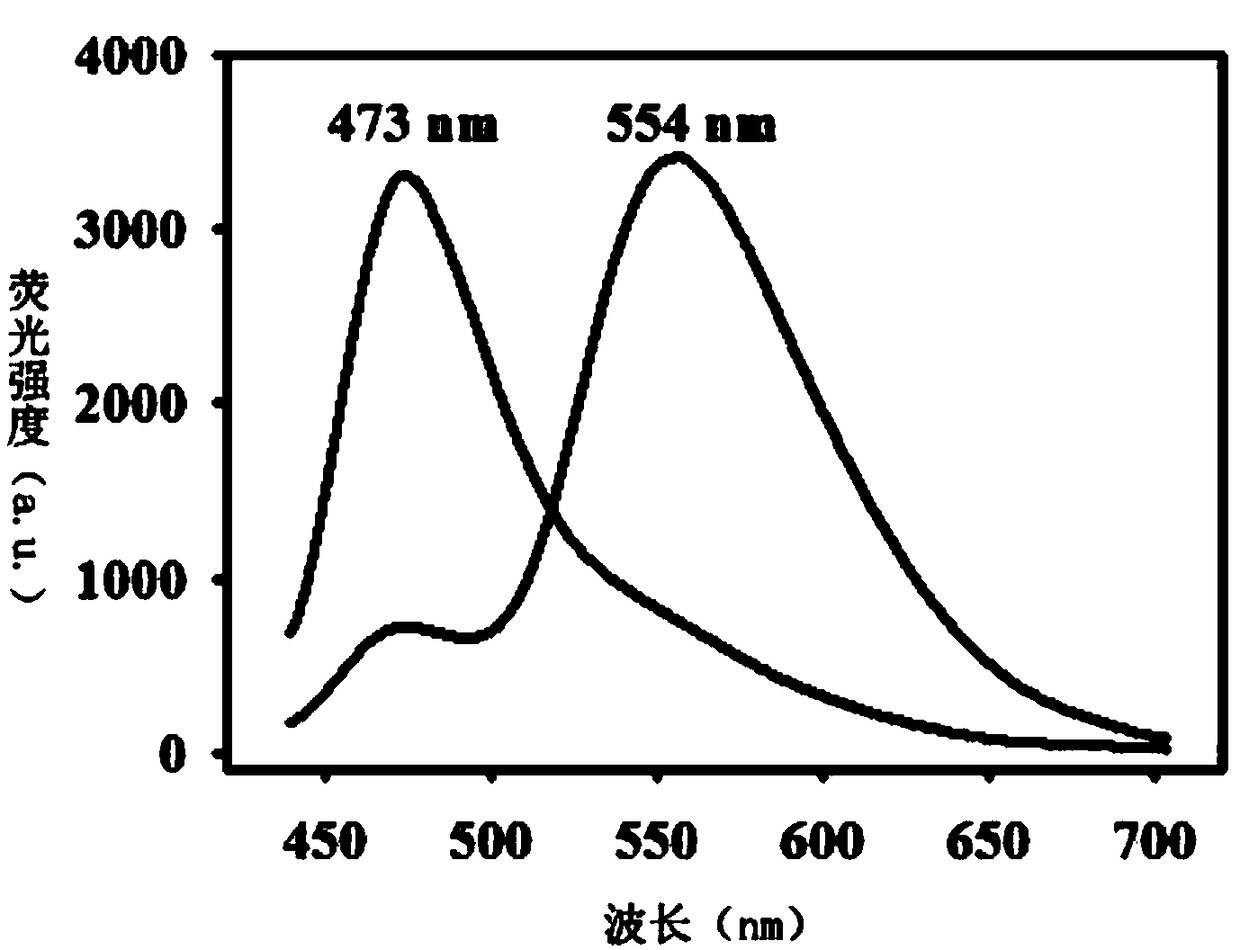
[0044] The NMR H spectrum of the fluorescent probe is:
[0045] 1 H NMR (50...
Embodiment 2
[0047] The experimental procedure of Example 2 is the same as that of Example 1, but the molar ratio of compound I and compound II is changed to 2:1 in step (1).
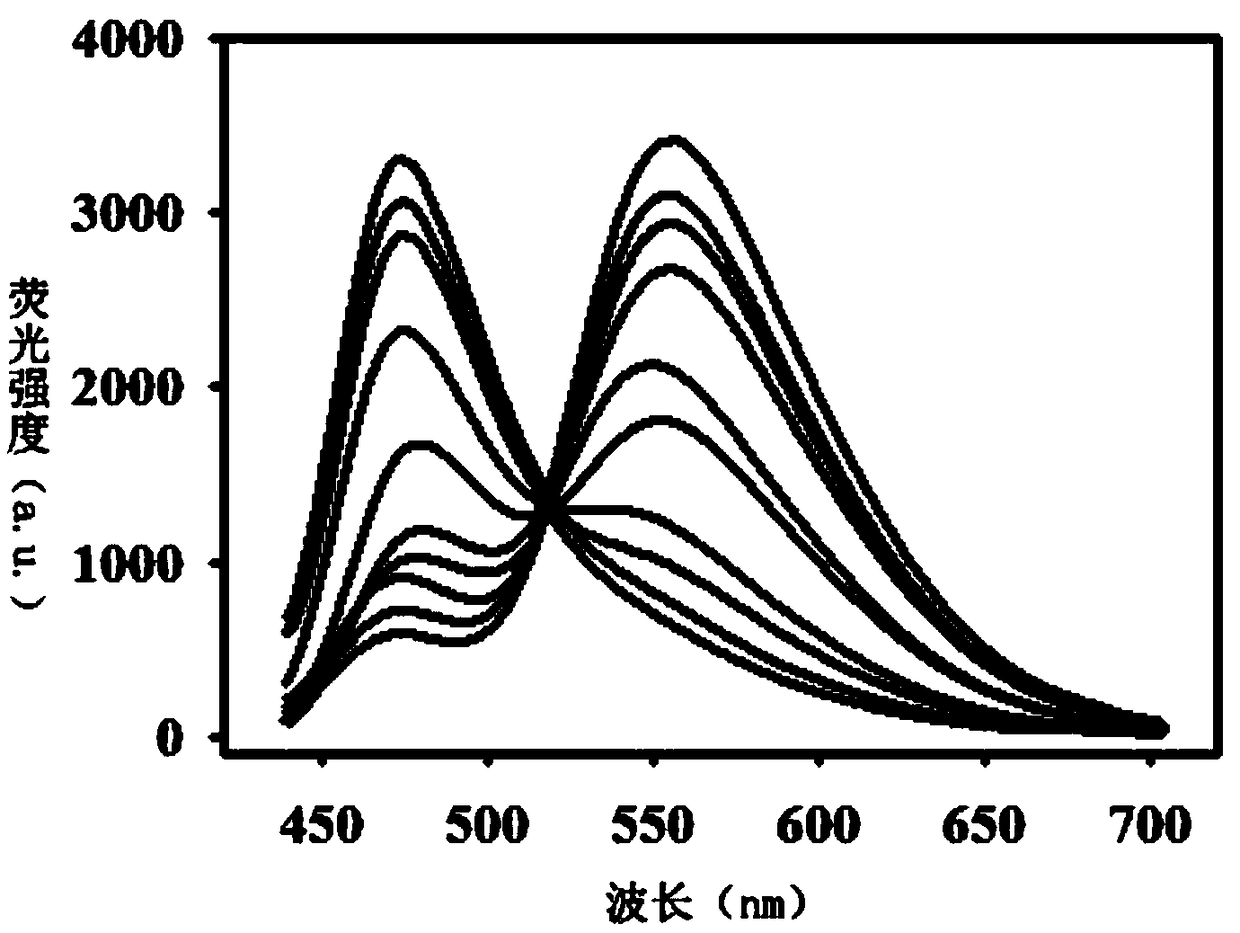
[0048] It was found through testing that the structure of the final product of Example 2 was consistent with that of Example 1, and the synthesis method of the probe of the present invention is not limited to the method described in the example.
Embodiment 3
[0050] The experimental procedure of Example 3 is the same as that of Example 1, but the molar ratio of compound I and compound II is changed to 1:2 in step (1).
[0051] It was found through testing that the structure of the final product of Example 3 was consistent with that of Example 1, and the synthesis method of the probe of the present invention is not limited to the method described in the example.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com