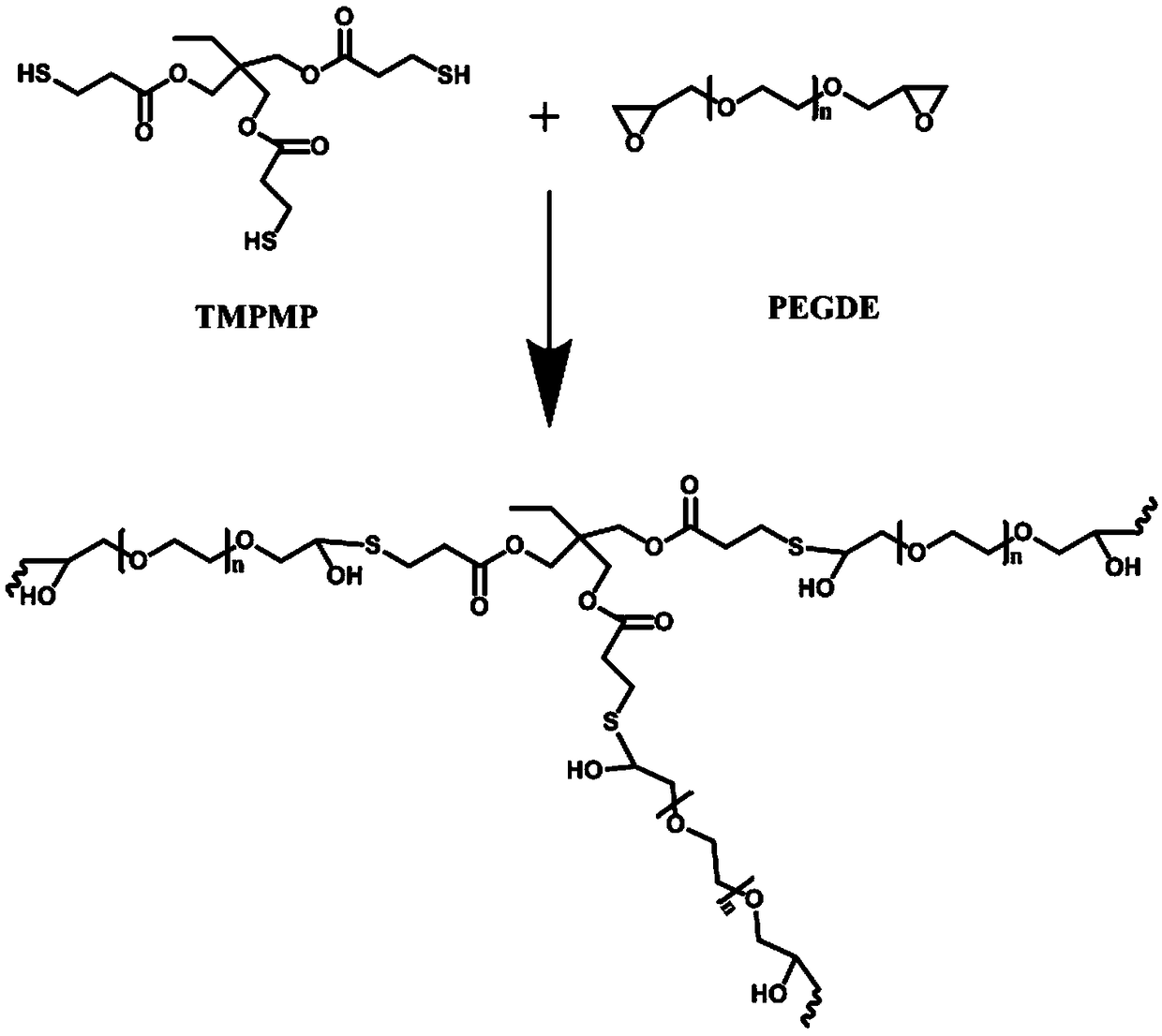
A crosslinked polymer electrolyte suitable for lithium secondary battery and a preparation method thereof
A cross-linked polymer, lithium secondary battery technology, applied in the field of electrolytes, can solve the problems of limited application, low room temperature ionic conductivity, etc., and achieve the effects of simple preparation, high room temperature conductivity, and good flexibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Polyethylene glycol diglycidyl ether (molecular weight 200) 4g, trimethylolpropane-tris(mercaptopropionate) 4g, add to the beaker, stir well, add 2.25g lithium trifluoromethanesulfonate, wait until completely dissolved Finally, 0.03 g of tetrabutylammonium fluoride was added, mixed evenly, coated on the glass surface, and heated at 70° C. for 4 hours to obtain a cross-linked polymer electrolyte membrane.
Embodiment 2
[0026] Polypropylene glycol diglycidyl ether (molecular weight 600) 5g, tetrakis (3-mercaptopropionate) pentaerythritol ester 3g, add to the beaker, after stirring evenly, add 4.7g of lithium perchlorate, after completely dissolving, add benzyl triethyl Mix 0.05 g of ammonium chloride evenly, apply it to the glass surface, and heat at 70° C. for 4 hours to obtain a cross-linked polymer electrolyte membrane.
Embodiment 3
[0028] Polyethylene glycol diglycidyl ether (molecular weight 1000) 5g, tetrakis (3-mercaptopropionate) pentaerythritol ester 4g, add to the beaker, after stirring evenly, add 8g of bistrifluoromethanesulfonimide lithium, after completely dissolving , add 0.08 g of triethylamine and mix evenly, apply it on the glass surface, and heat at 80°C for 2 hours to obtain a cross-linked polymer electrolyte membrane.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com