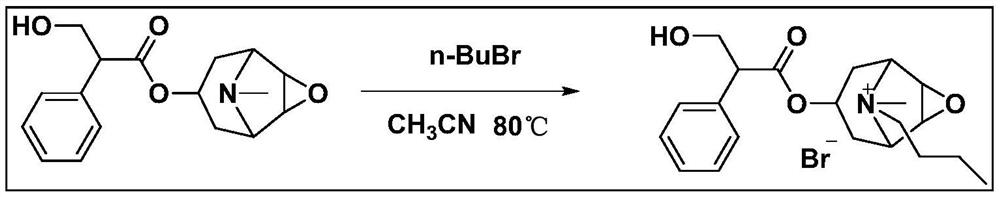
A kind of preparation method of medicinal scopolamine butylbromide
A technology of scopolamine and scopolamine, which is applied in the field of preparation of medicinal scopolamine butylbromide, can solve the problems of difficult post-processing, low yield, and the quality of finished products cannot meet the European Pharmacopoeia, and achieves less raw material residues, high yield, The effect of shortening the processing time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] An embodiment of the preparation method of the pharmaceutical butterchromatic alkalikine of the present invention, including the following steps:
[0029] (1) Preparation of buddus of butchromonoli: 5 g of Eastern alkali is dissolved in 2.5 ml of bromo n-butyl, then 1 ml of acetonitrile, heating at 65 ° C for 60 h; after the reaction is completed, the volume fraction of Eastern alkali weight is 0.5 times volume is 70%. Ethanol solution is concentrated under reduced pressure, and there is a crude bromide of butterolactone;
[0030] (2) Purification: The above-mentioned butchromodide is added to a methanol solution of 70%, after dissolving, filtration, placing room temperature, precipitated crystals in the refrigerator for 4 h, filtration, wash, and then dried The above operation was repeated, and the second, three, four-time recrystallization was carried out, and the drug was carried out at 70 ° C for 16 h at 70 ° C, i.e..
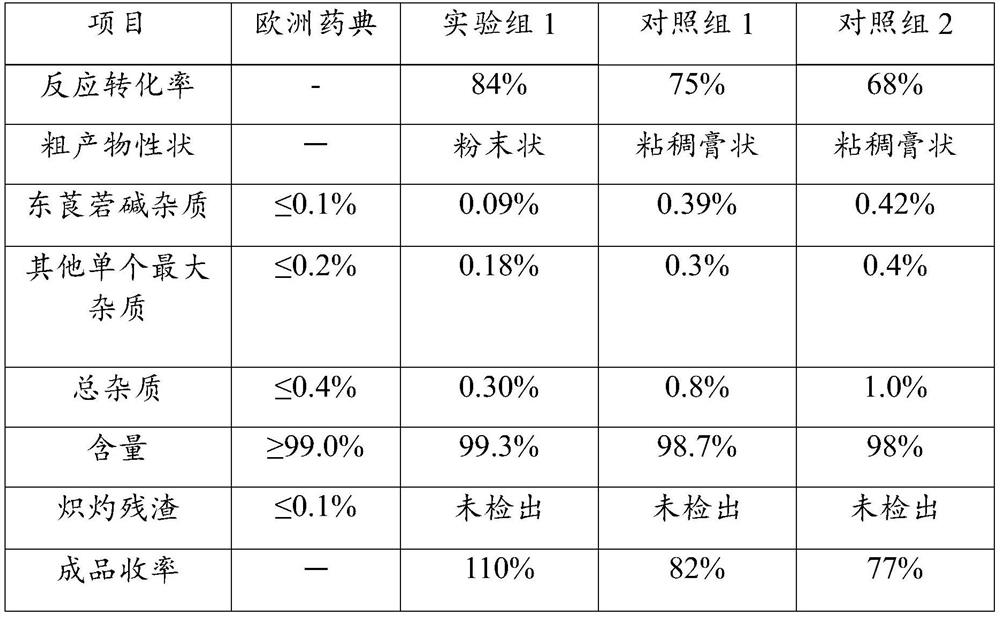
[0031] After testing, the product was 5.5 g, the y...
Embodiment 2
[0033] An embodiment of the preparation method of the pharmaceutical butterchromatic alkalikine of the present invention, including the following steps:
[0034] (1) Preparation of birundine of butchromoni: 5 g of Eastern alkali is dissolved in 7.5 ml of bromine n-butylation, then 10 ml of pyridine, heating at 80 ° C for 20 h; the reaction is completed, and the amount of anhydrous alkali is 5 times the volume of anhydrous ethanol is concentrated. , Deboratomodorine crude;
[0035] (2) Purification: The above-mentioned butchromodide is added to 25 ml of anhydrous methanol. After dissolving, it is filtered through heat, placed at room temperature, and precipitated crystals and placed in the refrigerator for 4 h, then dried at 100 ° C for 5 h, Medicine butterromide.
[0036] It was detected, and the product was 6 g, the yield was 120%, and the purity was 99.6%. Eastern is 0.07% of the bloodstatal mass, and other single maximum hetero mass of 0.16%, and the content is 99.6%.
Embodiment 3
[0038] An embodiment of the preparation method of the pharmaceutical butterchromatic alkalikine of the present invention, including the following steps:
[0039] (1) Preparation of birrochlorine alkaline: 20 g of east alkali is dissolved in 30 ml of bromine n-butyne, then 20 mL of acetonitrile, heating at 80 ° C for 35 h; the reaction ends, the volume fraction of the eastiorine base is 90% methanol The solution was concentrated under reduced pressure, and there was a crude bromoantine.
[0040] (2) Purification: The above-mentioned butchromodiochioline is added to the ethanol solution of 40 ml of volume fraction. After dissolving, it is filtered through heat, placed at room temperature, precipitated crystals in the refrigerator for 4 h, filtered, wash and dried The above operation was repeated in the addition of 75% of the ethanol solution, and the second recrystallization was performed, and the drug was dried in vacuo to dry at 100 ° C, i.e.
[0041] After testing, 23.6 g of the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com