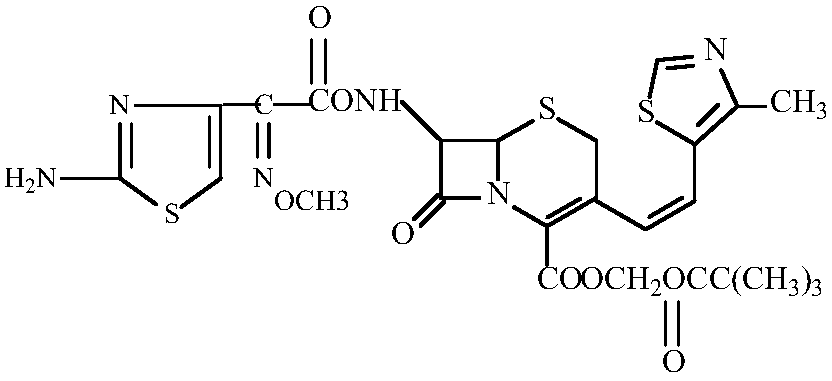
Preparation method of amorphous cefditoren pivoxil
A cefditoren axetil and amorphous technology, applied in the field of preparation of amorphous cefditoren axetil, can solve problems such as unreported cefditoren axetil, and achieve the effects of stable and feasible process, good product quality and high solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
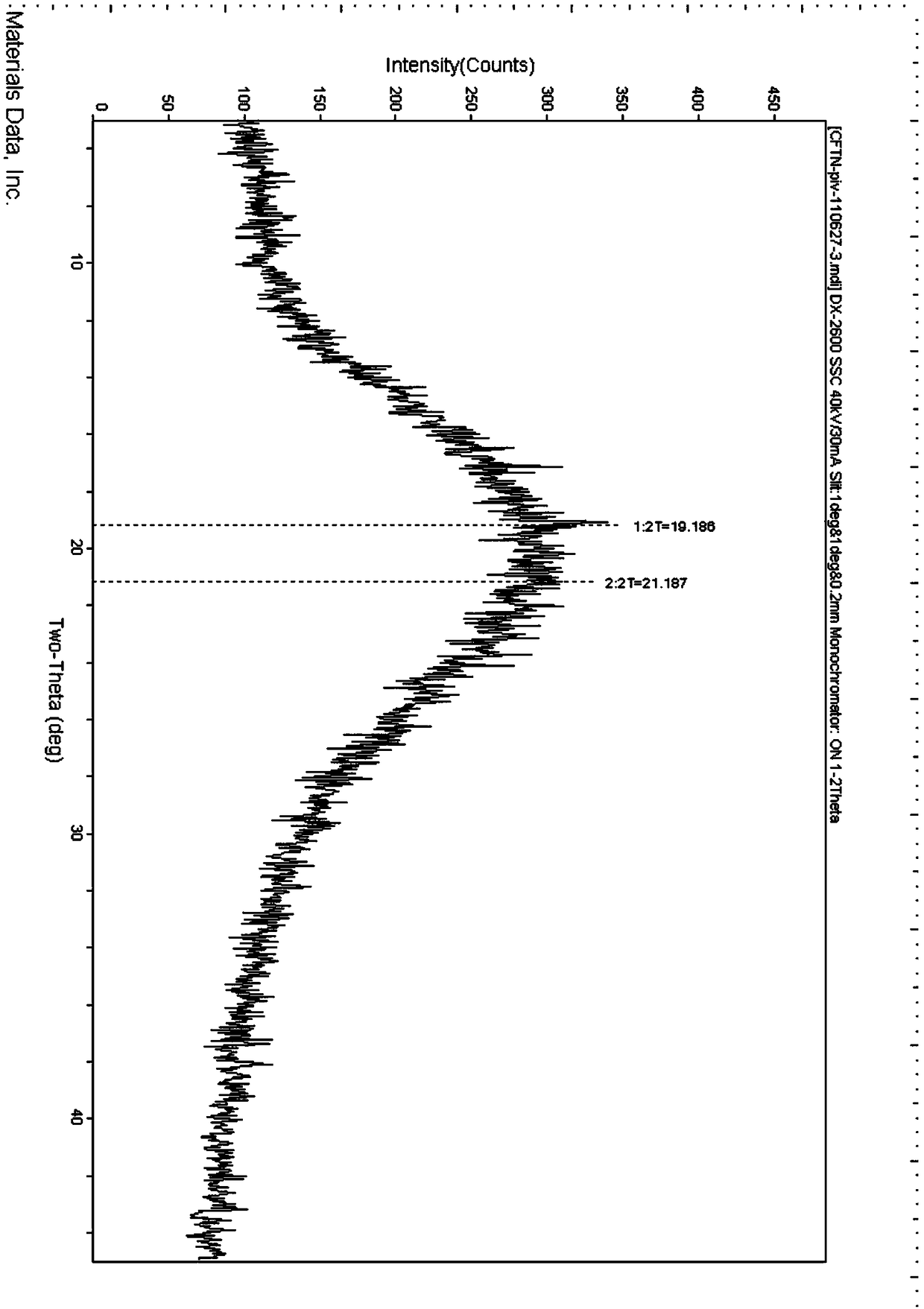
[0021] Suspend 50 grams of cefditoren axetil in 500 milliliters of dichloromethane, add 5 grams of activated carbon, heat up and stir for 30 minutes, filter, and wash the activated carbon with a small amount of dichloromethane. The filtrate and washing liquid were combined, concentrated to dryness under reduced pressure at 35°C, and the obtained solid was detected by XPRD as amorphous.
Embodiment 2
[0023] Suspend 50 grams of cefditoren axetil in 200 milliliters of dichloromethane, add 300 milliliters of acetone, then add 5 grams of activated carbon, heat up and stir for 30 minutes, filter, and wash the activated carbon with a small amount of dichloromethane. The filtrate and washing liquid were combined, concentrated under reduced pressure at 35°C to about 100 ml, added dropwise into 1000 ml of ether, precipitated, cooled in an ice bath at 0-5°C, stirred for 1 hour, filtered, washed with ether, and dried in vacuo. The resulting solid was detected by XPRD and was amorphous.
Embodiment 3
[0025] Suspend 50 grams of cefditoren axetil in 500 milliliters of dichloromethane, add 5 grams of activated carbon, heat up and stir for 30 minutes, filter, and wash the activated carbon with a small amount of dichloromethane. The filtrate and washing liquid were combined, concentrated under reduced pressure at 35°C to 100 ml, added dropwise into 1000 ml of ether, precipitated, cooled in an ice bath at 0-5°C, stirred for 1 hour, filtered, washed with ether, and dried in vacuo. Gained solid is detected by XPRD, such as figure 1 Shown is amorphous.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com