Method for preparing beta-niacinamide single nucleotide or beta-niacinamide ribose
A technology of nicotinamide ribose and mononucleotide, which is applied in the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc., can solve the problems of difficult reaction purification, low yield, and difficult amplification.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
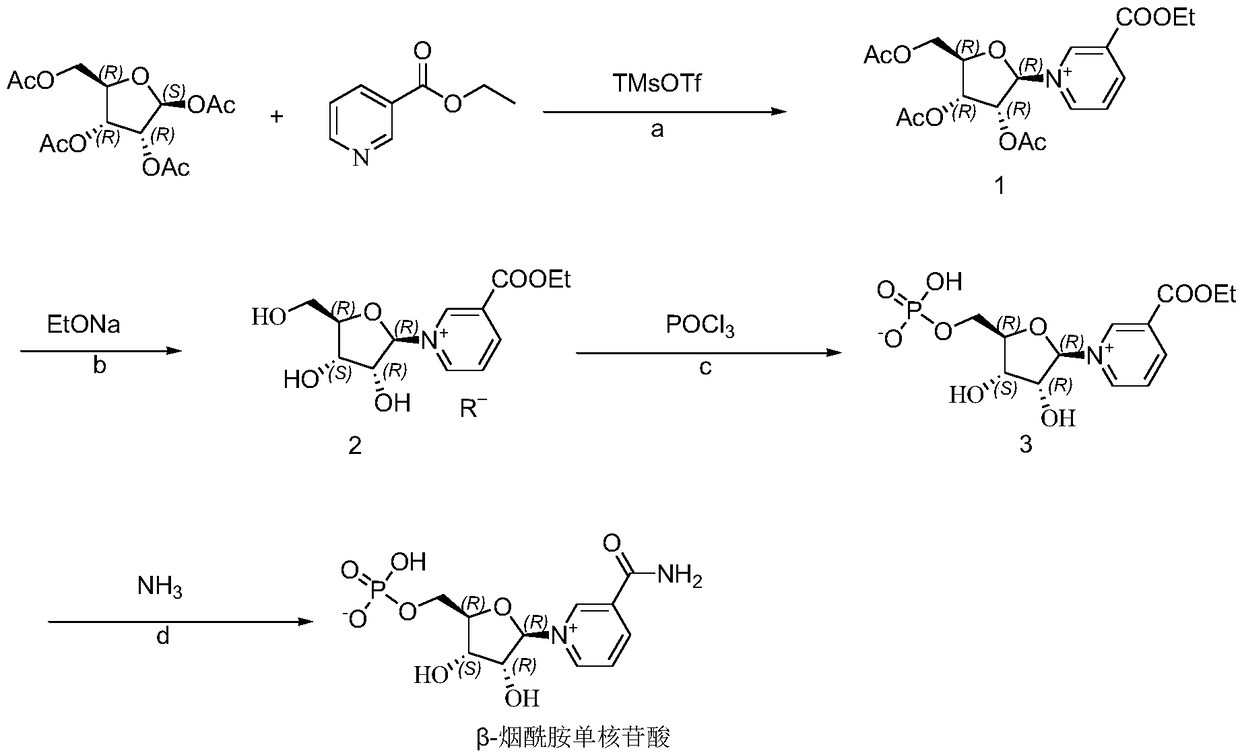
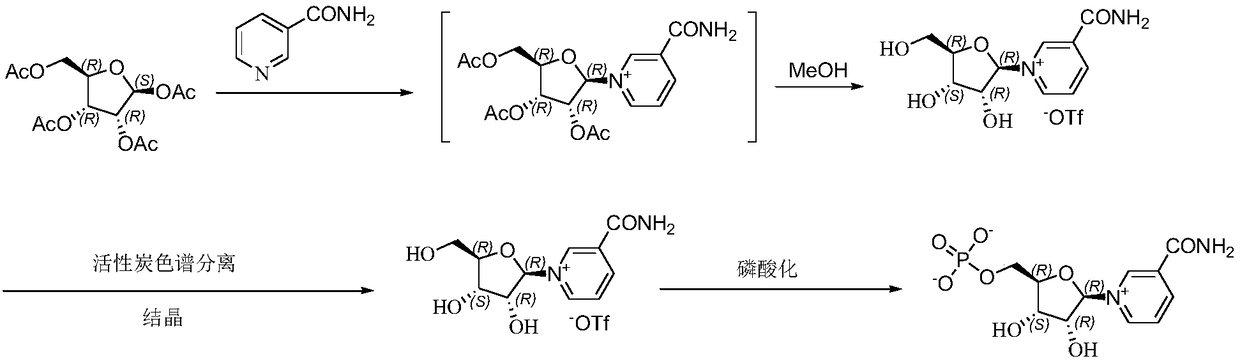
[0085] Example 1: Preparation of β-nicotinamide mononucleotide
[0086] a. Condensation (that is, preparation of nicotinic acid ethyl ester triacetyl riboside):
[0087] In a 1L three-necked flask, add tetraacetyl ribose (20g, 62.8mmol), 20mL of dichloromethane, stir and dissolve, then add ethyl nicotinate (14.3g, 94.3mmol), stir well; at room temperature, add dropwise with 20mL of dichloromethane Diluted trimethylsilyl trifluoromethanesulfonate (TMsOTf 21g, 94.3mmol) was exothermic during the dropwise addition, the temperature was controlled below 40°C, the addition was completed within about 0.5 hours, the dropwise addition was completed, and then the reaction was stirred for 0.5-1 hours to complete the reaction (elimination of tetraacetyl ribose monitored by TLC). After the reaction was completed, the reaction solution was cooled to below -5°C, 10 mL of ethanol was added dropwise, and the reaction was terminated by stirring for 15 min to destroy the TMSOTf quenching reacti...
Embodiment 2
[0095] Example 2: Preparation of β-nicotinamide mononucleotide
[0096] a. Condensation (that is, preparation of nicotinic acid ethyl ester triacetyl riboside):
[0097] Add tetraacetyl ribose (10g, 31.4mmol) and 10mL of dichloromethane to a 1L three-necked flask, stir and dissolve, then add ethyl nicotinate (7.1g, 47.1mmol), stir well; at room temperature, add dropwise 10mL of dichloromethane Diluted trimethylsilyl trifluoromethanesulfonate (TMsOTf 10.5 g, 47.1 mmol) was exothermic during the dropwise addition, and the temperature was controlled below 40°C, the addition was completed within about 0.5 hours, the dropwise addition was completed, and then the reaction was stirred for 0.5~ 1 hour to complete the reaction (elimination of tetraacetyl ribose monitored by TLC). After the reaction was completed, the reaction solution was cooled to below -5°C, 5 mL of ethanol was added dropwise, and the reaction was terminated by stirring for 15 min to destroy the TMSOTf quenching rea...
Embodiment 3
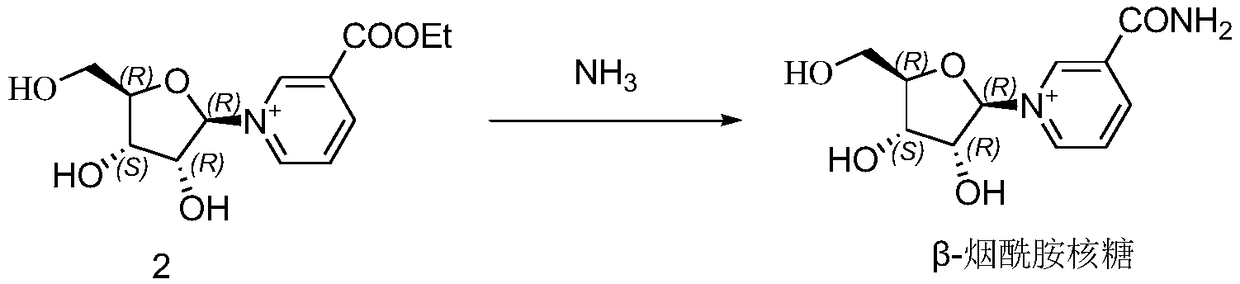
[0105] Example 3: Preparation of β-nicotinamide ribose
[0106] a. Condensation (that is, preparation of nicotinic acid ethyl ester triacetyl riboside):
[0107] In a 1L three-necked flask, add tetraacetyl ribose (20g, 62.8mmol), 20mL of dichloromethane, stir and dissolve, then add ethyl nicotinate (14.3g, 94.3mmol), stir well; at room temperature, add dropwise with 20mL of dichloromethane Diluted trimethylsilyl trifluoromethanesulfonate (TMsOTf 21g, 94.3mmol) was exothermic during the dropwise addition, the temperature was controlled below 40°C, the addition was completed within about 0.5 hours, the dropwise addition was completed, and then the reaction was stirred for 0.5-1 hours to complete the reaction (elimination of tetraacetyl ribose monitored by TLC). After the reaction was completed, the reaction solution was cooled to below -5°C, 10 mL of ethanol was added dropwise, and the reaction was terminated by stirring for 15 min to destroy the excess TMSOTf to quench the rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com