A human venous thrombosis risk gene f5 and pai-1 polymorphism detection kit and its preparation method and application
A detection kit, PAI-1 technology, applied in the field of biomedical clinical detection, can solve the problems of low detection sensitivity, unfavorable clinical promotion, high equipment requirements, etc., to improve detection efficiency, avoid false negative and false positive problems, The effect of ensuring accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
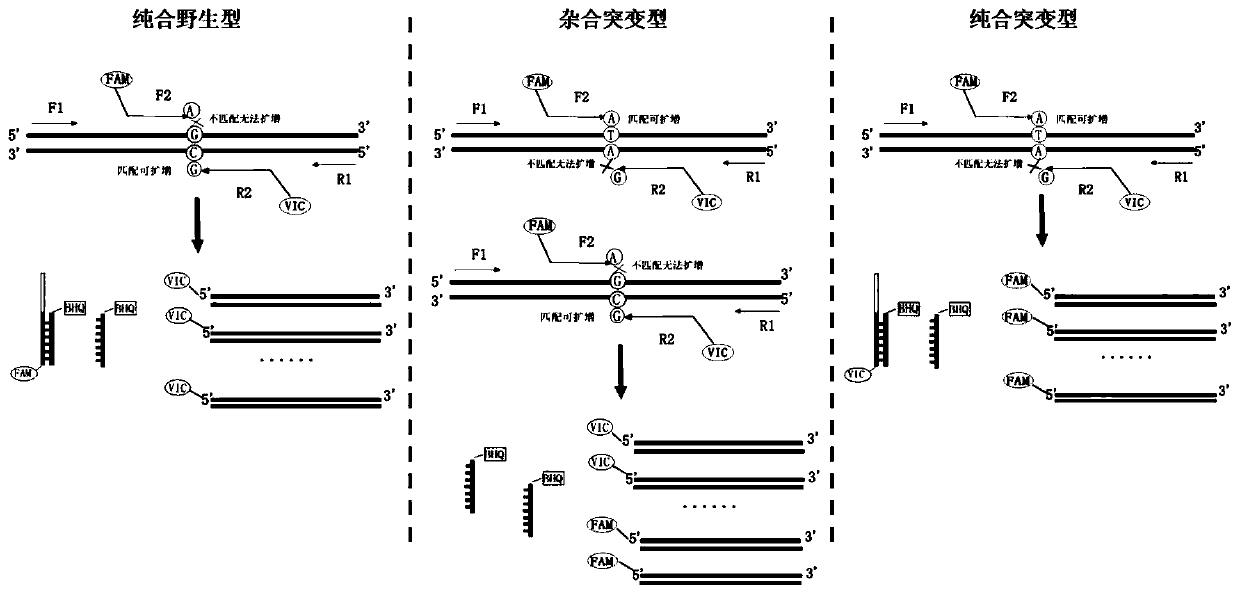
[0047] A human venous thrombosis risk gene F5 and PAI-1 polymorphism detection kit, including detection reagent 1, detection reagent 2, positive control substance and negative control substance; the detection reagent 1 is aimed at the rs6025 polymorphism site of F5 gene , comprising: a forward primer whose sequence is shown in SEQ ID NO.1, a reverse primer whose sequence is shown in SEQ ID NO.2, a sequence shown in SEQ ID NO.3 and identification of a VIC fluorescent group at the 5' end Primer, sequence shown in SEQ ID NO.4 and 5' end carrying FAM fluorescent group identification primer, sequence shown in SEQ ID NO.5 and 3' end carrying BHQ quenching group PNA, sequence as SEQ ID NO .6 and the 3' end carries the PNA of the BHQ quenching group, the internal reference forward primer whose sequence is shown in SEQ ID NO.7, the internal reference reverse primer whose sequence is shown in SEQ ID NO.8, and the sequence such as SEQ ID NO.8 Internal reference identification primer show...
Embodiment 2
[0074] A human venous thrombosis risk gene F5 and PAI-1 polymorphism detection kit, including detection reagent 1, detection reagent 2, positive control and negative control; the components of the detection reagent 1 and detection reagent 2 are as follows in Table 1 , the composition of the kit is shown in Table 2:
[0075] Table 1 detection reagent composition
[0076]
[0077]
[0078] Table 2 Kit Composition
[0079]
Embodiment 3
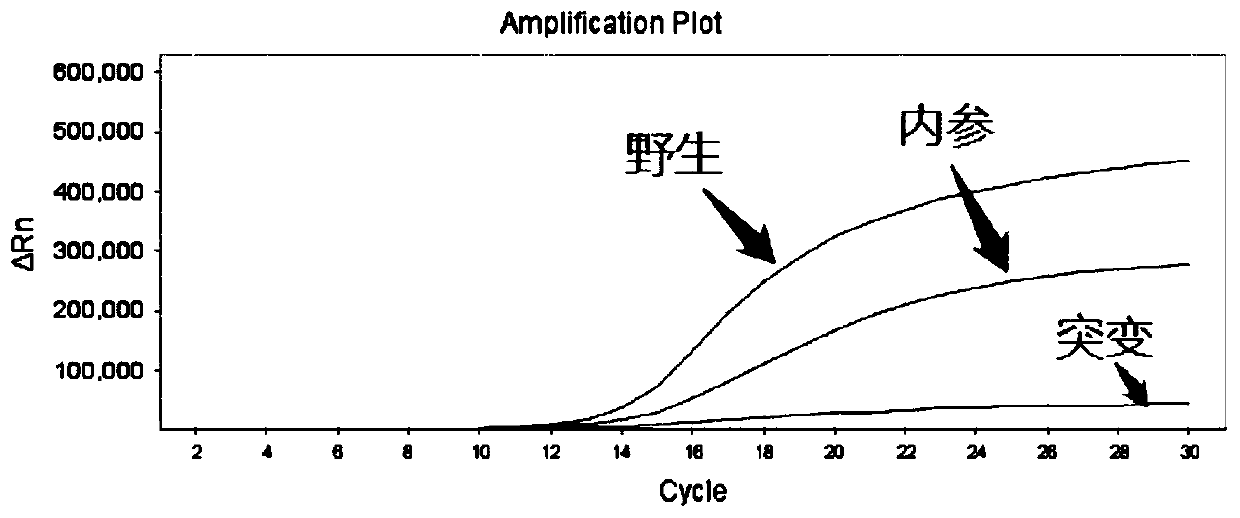
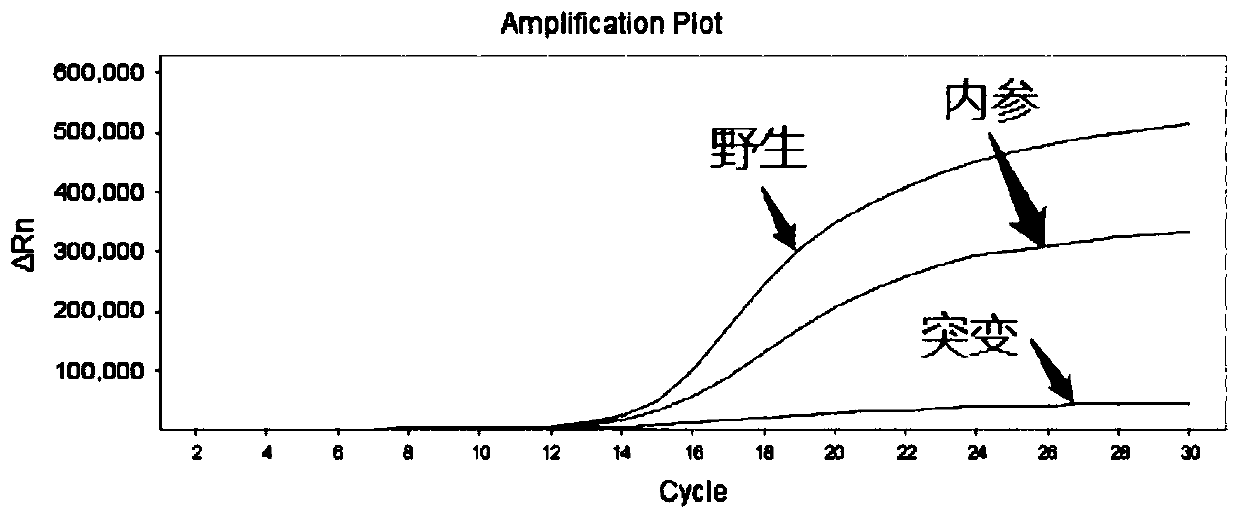
[0081] The minimum detection limit of the sample detected using the kit prepared in Example 2 specifically includes the following steps:
[0082] (1) Use Tiangen Biochemical Biotechnology Company Whole Blood DNA Extraction Kit to extract 5 human peripheral blood genomic DNAs. The specific operation steps are strictly in accordance with the kit instructions to obtain human genomic DNA samples to be tested, and dilute the samples to 2ng / μL , 0.5ng / μL, 0.1ng / μL, 0.05ng / μL.
[0083] (2) Take PCR eight-tubes, add 20 μL of gradient dilution samples to each well; add 30 μL of well-mixed F5 detection reagent or 30 μL of PAI-1 detection reagent to each well; shake and centrifuge the eight-tubes to mix The reaction solution;
[0084] (3) Put the eight tubes into the ABI 7500 fluorescent PCR instrument for amplification detection. The PCR reaction conditions are shown in Table 3:
[0085] Table 3 PCR reaction program
[0086]
[0087] (4) Analysis results:
[0088] Analyzing the t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com