Anti-androgen effect detection method
A detection method, an anti-androgen technology, applied in the field of detection of anti-androgen effects, can solve the problems of high cost of time and money, large error in test results, and many interference factors, and achieve simplified operation, high continuous stability, and reduced cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
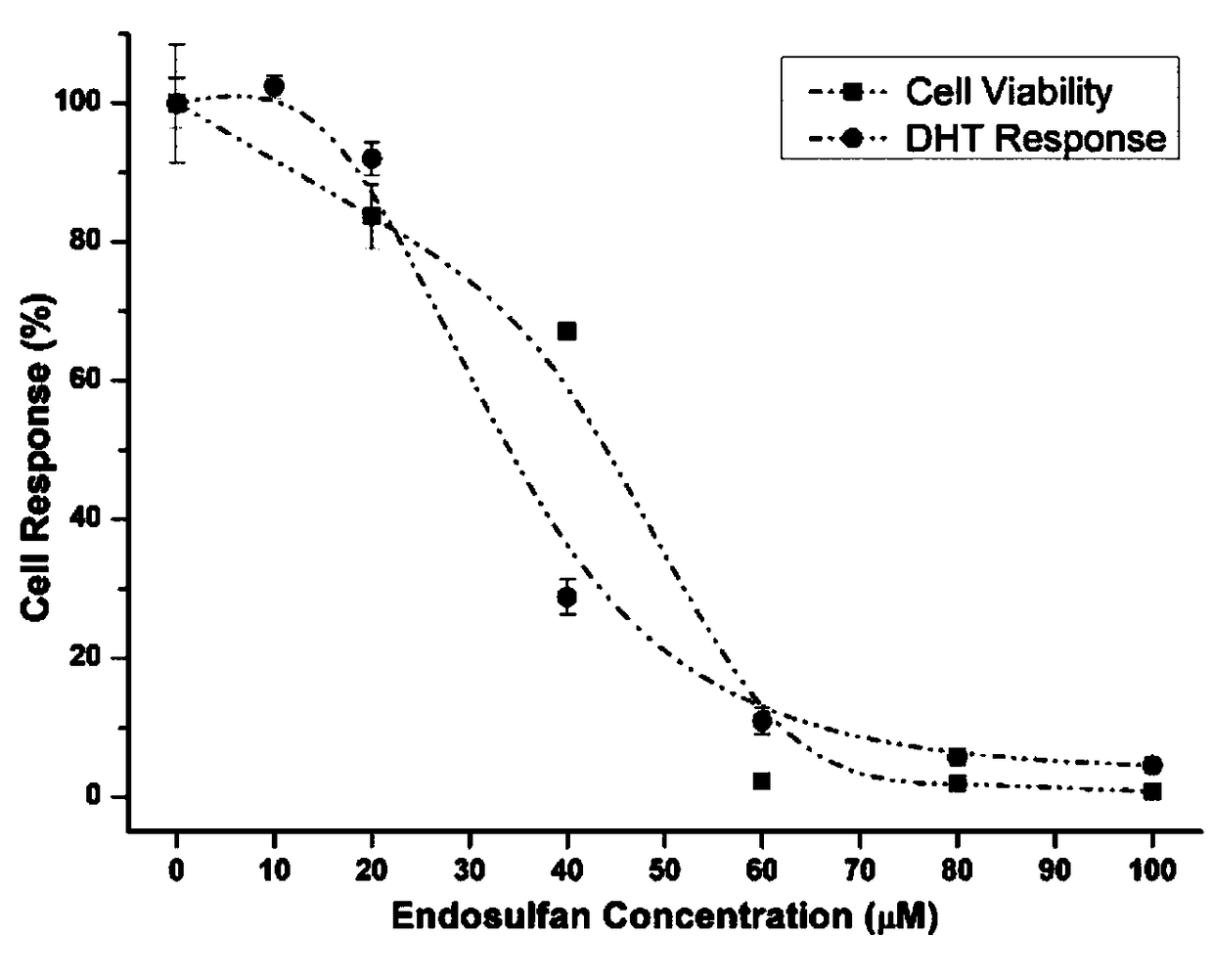
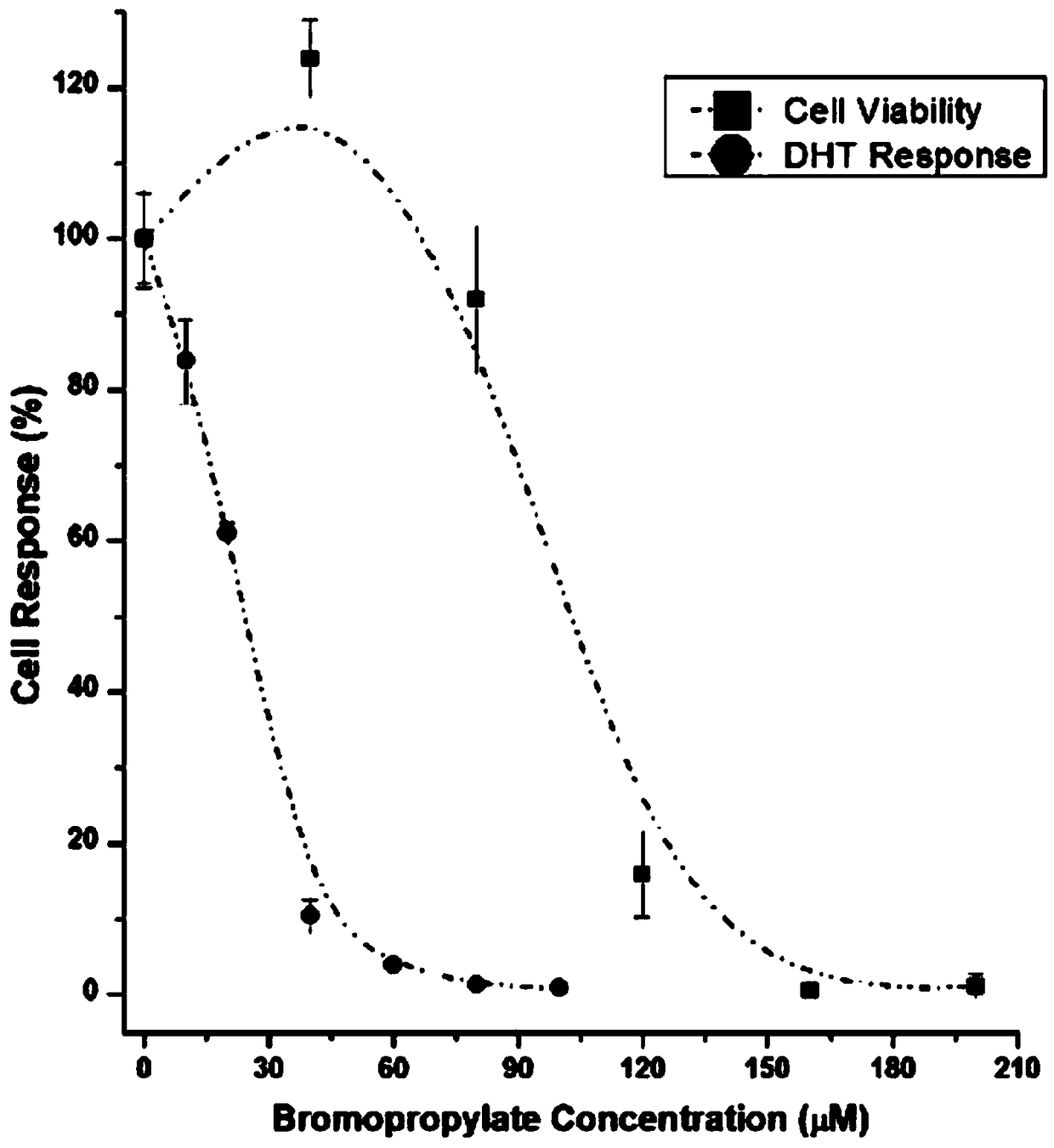
[0095] 1. Determination of cytotoxic activity by MTT colorimetry:
[0096] 1. Adjust the cell density with L-15 medium containing 10% fetal bovine serum, 1×10 per hole in 100ul 4 Cells were seeded into 96-well culture plates at 37°C, 5% CO 2 Incubate for 24 h under conditions.
[0097] 2. Remove the culture medium, add 100ul of pesticide-containing medium without fetal bovine serum, and 0.1% DMSO as a blank control group.
[0098] 3. Incubate with pesticides for 24 hours, and remove the drug-containing medium.
[0099] 4. Add 110ul 10% MTT solution, prepare it with serum-free medium, and incubate for 3h.
[0100] 5. Detect the absorbance value of each well at 490nm with a microplate reader.
[0101] Cell viability was calculated using the formula:
[0102] Cell Viability / %=(OD sample-OD blank) / (OD control-OD blank)×100.
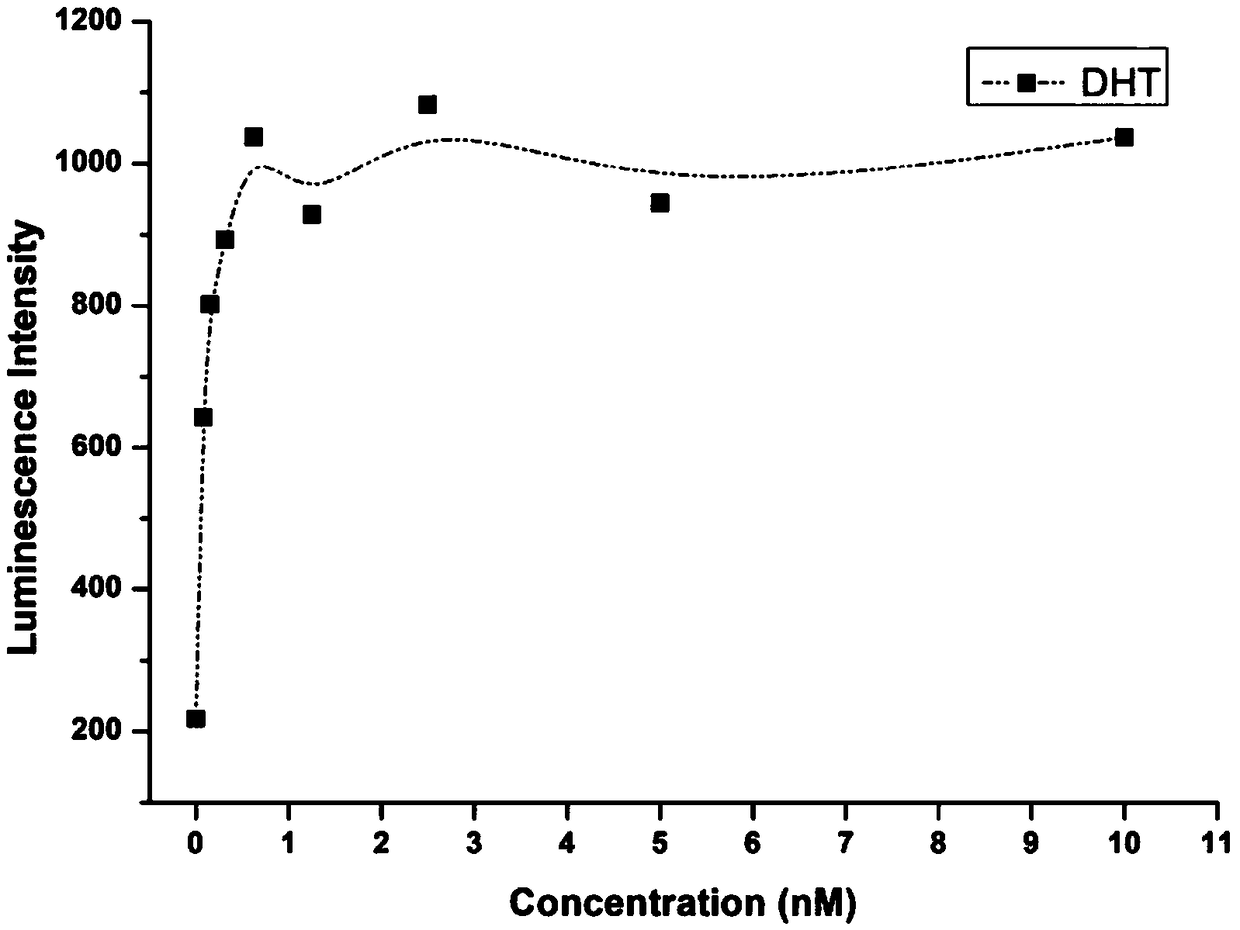
[0103] 2. Determination method of anti-androgen effect of pesticides:
[0104] 1. Using the MDA-kb2 breast cancer cell line, adjust the cell density w...
Embodiment 2
[0111] 1. Determination of cytotoxic activity by MTT colorimetry:
[0112] 1. Adjust the cell density with L-15 medium containing 9% fetal bovine serum, 0.8×10 per hole in 100ul 4 Cells were seeded into 96-well culture plates at 37°C, 5% CO 2 Incubate for 23 h under conditions.
[0113] 2. Remove the culture medium, add 100ul of pesticide-containing medium without fetal bovine serum, and 0.1% DMSO as a blank control group.
[0114] 3. Incubate with pesticides for 24 hours, and remove the drug-containing medium.
[0115] 4. Add 110ul 10% MTT solution, prepare it with serum-free medium, and incubate for 3h.
[0116] 5. Detect the absorbance value of each well at 490nm with a microplate reader.
[0117] Cell viability was calculated using the formula:
[0118] Cell Viability / %=(OD sample-OD blank) / (OD control-OD blank)×100.
[0119] 2. Determination method of anti-androgen effect of pesticides:
[0120] 1. Using the MDA-kb2 breast cancer cell line, adjust the cell density ...
Embodiment 3
[0127] 1. Determination of cytotoxic activity by MTT colorimetry:
[0128] 1. Adjust the cell density with L-15 medium containing 11% fetal bovine serum, 1.2×10 per hole in 100ul 4 Cells were seeded into 96-well culture plates at 37°C, 5% CO 2 Incubate for 26 h under conditions.
[0129] 2. Remove the culture medium, add 100ul of pesticide-containing medium without fetal bovine serum, and 0.1% DMSO as a blank control group.
[0130] 3. Incubate with pesticides for 24 hours, and remove the drug-containing medium.
[0131] 4. Add 110ul 10% MTT solution, prepare it with serum-free medium, and incubate for 3h.
[0132] 5. Detect the absorbance value of each well at 490nm with a microplate reader.
[0133] Cell viability was calculated using the formula:
[0134] Cell Viability / %=(OD sample-OD blank) / (OD control-OD blank)×100.
[0135] 1. Using the MDA-kb2 breast cancer cell line, adjust the cell density with L-15 medium containing 11% fetal bovine serum to contain 1.2×10 4 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com