A kind of lacrimal canaliculus slow-release hydrogel implant and preparation method thereof
An implant and hydrogel technology, applied in the field of medicine, can solve the problems of difficulty in maintaining a stretched state, poor tensile properties of silicone tubes, and difficulties in industrialized production, and achieve high stretching efficiency, small batch-to-batch variation, and productivity. big effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0053] A preparation method of a lacrimal canalicular slow-release hydrogel implant of the present invention, comprising the following steps:
[0054] (1) Micronize the active ingredient raw material to obtain active ingredient micropowder, and dissolve the color developing agent in borax buffer;
[0055] (2) Dissolving the polyethylene glycol derivative with borax buffer solution, then adding the active ingredient micropowder, and stirring to form a uniform active ingredient suspension;
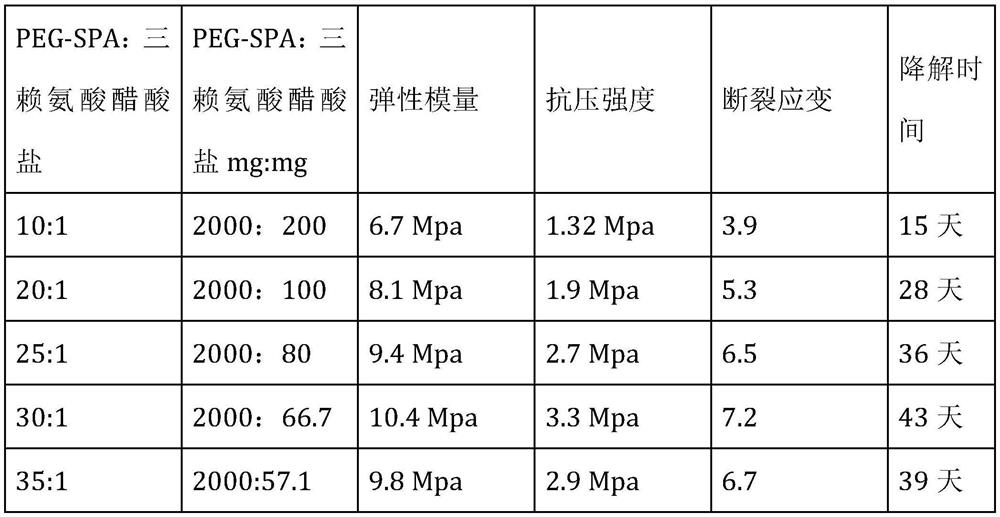
[0056] (3) Trilysine acetate is dissolved in borax buffer solution, then added to the active ingredient suspension, stirred and mixed evenly;
[0057] (4) Pour the mixed suspension into the silica gel tube, let it stand, and form a hydrogel after chemical crosslinking;
[0058] (5) The hydrogel is vacuum-dried to a constant weight, and then taken out and placed in a high-humidity environment, which absorbs moisture to increase in weight and become soft;
[0059] (6) After moisture absorpti...
Embodiment 1
[0066] Embodiment 1: the preparation of dexamethasone implant
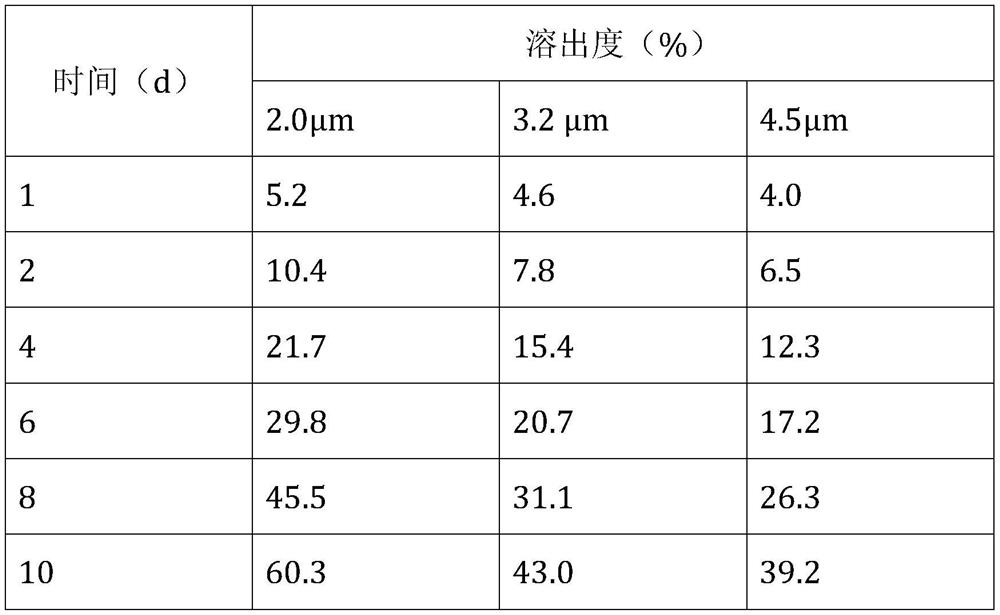
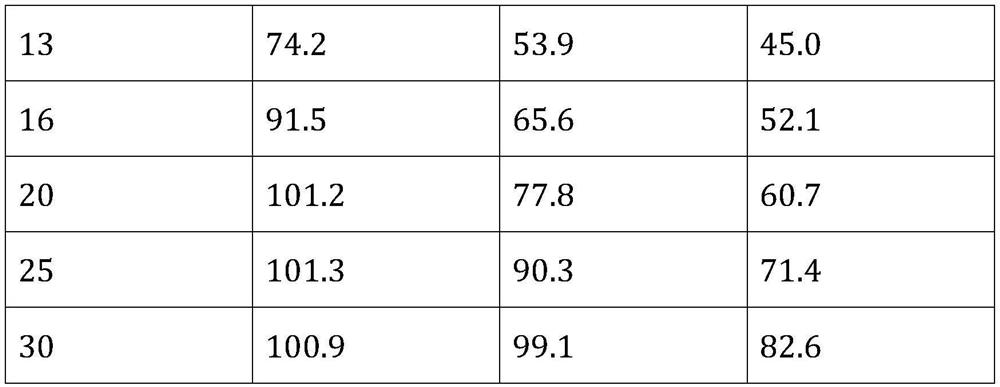
[0067] (1) The dexamethasone raw material is air-pulverized, and the average particle size of the obtained micropowder is 3.2 μm, wherein 90% of the particles are less than 5 μm;
[0068] (2) Add 2000mg of 4-arm polyethylene glycol succinimidyl propionate (PEG-SPA, molecular weight 10K) to 20mL of 0.015M borax buffer, stir to dissolve, then add 0.5mL of Tween with a concentration of 1mg / mL 80 aqueous solution, then add 230 mg active ingredient micropowder, and stir to form a uniform suspension;
[0069] (3) Dissolve 70mg of trilysine acetate in 5mL of 0.015M borax buffer, then add it to the active ingredient suspension, stir and mix evenly;
[0070] (4) Inject the mixed suspension into the silica gel tube, let it stand, and form a hydrogel after chemical crosslinking;
[0071] (5) The hydrogel is vacuum-dried to a constant weight, and then placed in a 92.5% RH high-humidity environment to absorb moisture and inc...
Embodiment 2
[0073] Embodiment 2: the preparation of cyclosporine implant
[0074] (1) The cyclosporine raw material is air-pulverized, and the average particle size of the obtained micropowder is 5.5 μm, wherein 90% of the particles are smaller than 10 μm;
[0075] (2) Add 3000mg of 8-arm polyethylene glycol succinimidyl glutarate (molecular weight 15K) to 15mL of 0.005M borax buffer, stir to dissolve, then add 1mL of Povidone K30 aqueous solution with a concentration of 0.7mg / mL , then add 175mg active ingredient micropowder, stir to form a homogeneous suspension;
[0076] (3) Dissolve 100mg trilysine acetate in 10mL of 0.005M borax buffer, then add it to the active ingredient suspension, stir and mix evenly;
[0077] (4) Inject the mixed suspension into the silica gel tube, let it stand, and form a hydrogel after chemical crosslinking;
[0078] (5) The hydrogel is vacuum-dried to a constant weight, and then placed in a 92.5% RH high-humidity environment to absorb moisture and gain 40%...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com