Pharmaceutic preparation of palbociclib and preparation method of pharmaceutic preparation
一种药用盐、无定型的技术,应用在帕布昔利布的药物制剂及其制备领域,能够解决病人吸收差等问题,达到增加体外溶出、增加生物利用度的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Preparation of dosage forms of acidic excipients and palbociclib dry granulated filling capsules
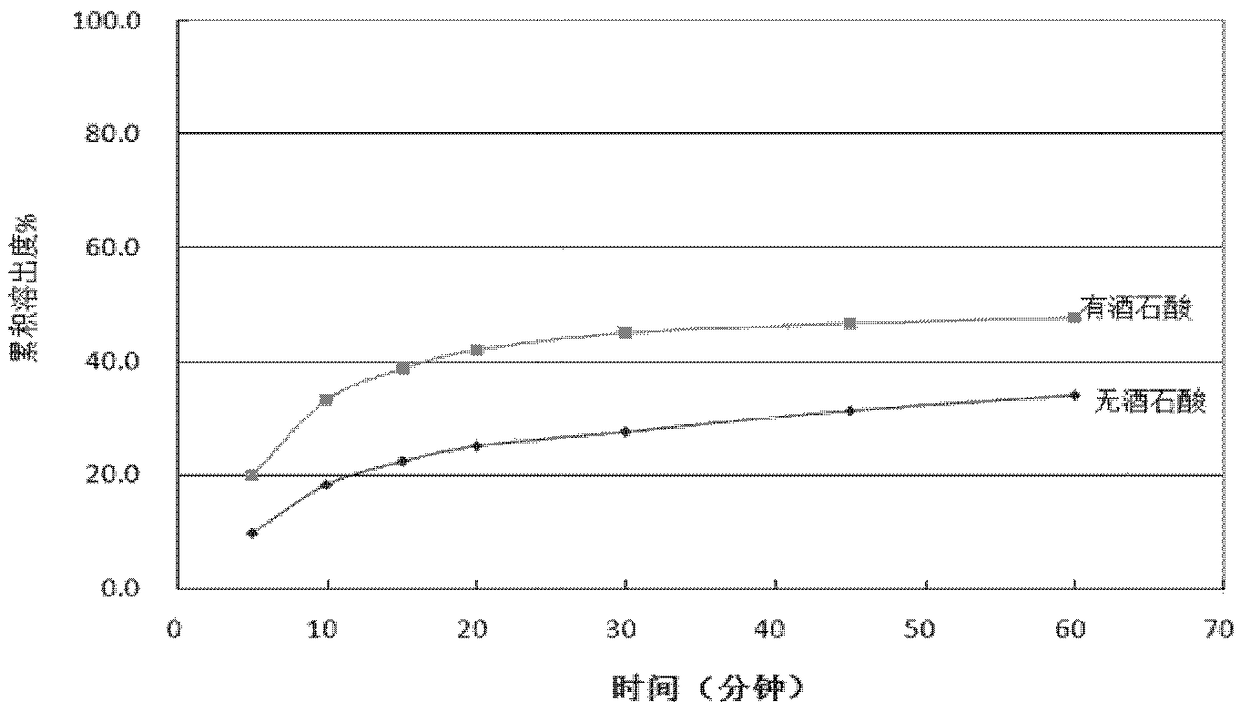
[0057] Take by weighing 1500 mg of palbociclib bulk drug, 345 mg of lactose, 1200 mg of tartaric acid, 840 mg of microcrystalline cellulose, 210 mg of sodium carboxymethyl starch, and 84 mg of silicon dioxide, mixed for 15 minutes with a three-dimensional mixer, and then Add 21 mg of magnesium stearate, and mix for another 2 minutes with a three-dimensional mixer. After mixing, the materials were passed through a 40-mesh sieve, and compressed with a mechanical single-punch tablet press. The pressure of the compression was 5 MPa, and each tablet weighed 1050 mg. Break the pressed large tablets, pass through a 10-mesh sieve, and pack in gelatin capsules, each capsule is loaded with 350 mg of medicine. Dissolution was measured at pH 6.0 at selected time points of 5, 10, 15, 20, 30, 45 and 60 minutes.
[0058] At the same time, a comparative test without acidic excipients wa...
Embodiment 2
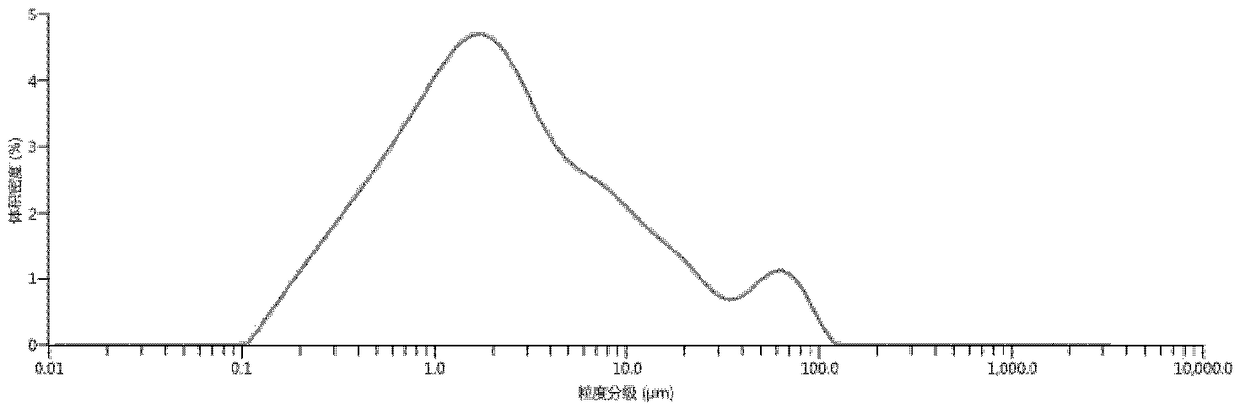
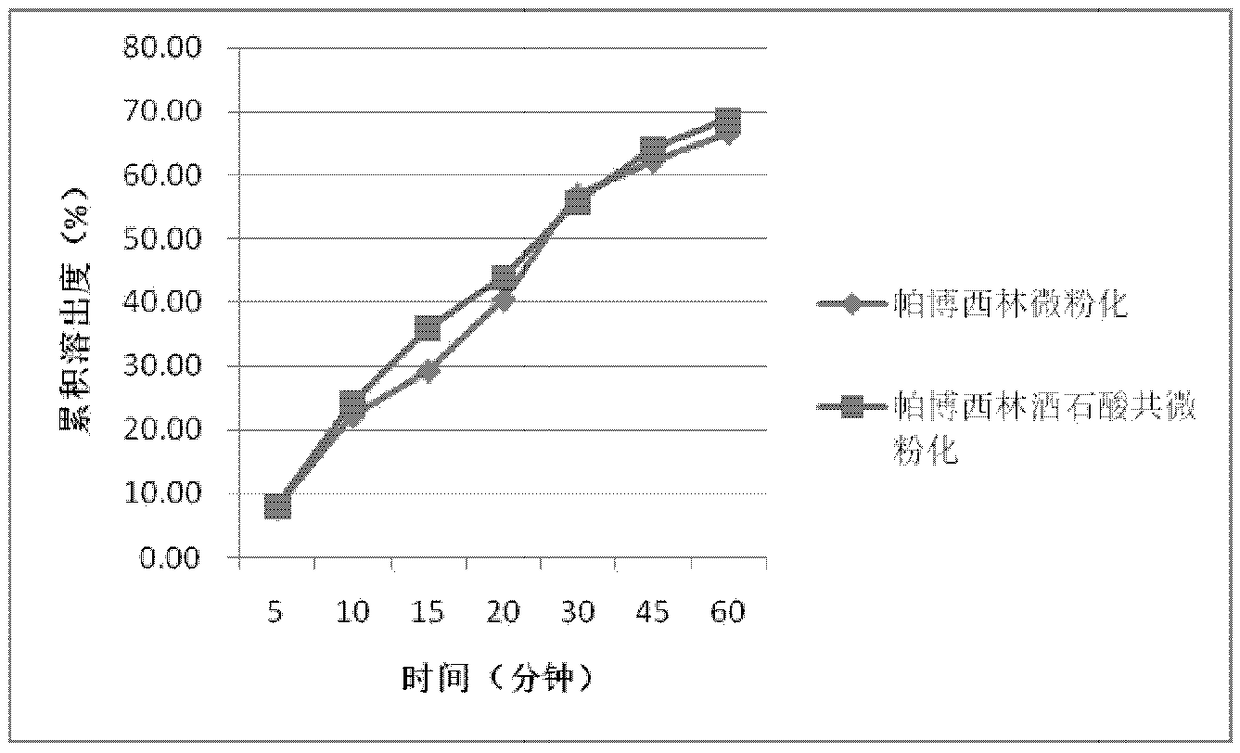
[0061] Preparation of dry granulated filling capsules after mixing acidic materials with palbociclib and crushing
[0062] Weigh 1500 mg of palbociclib bulk drug that has been jet-milled, and then mix it with 1200 mg of tartaric acid, 345 mg of lactose, 840 mg of microcrystalline cellulose, 210 mg of sodium carboxymethyl starch, and 84 mg of silicon dioxide in a three-dimensional Mix in a mixer for 15 minutes. Add 21 mg of magnesium stearate, and mix for another 2 minutes with a three-dimensional mixer. After mixing, the materials were passed through a 40-mesh sieve, and compressed with a mechanical single-punch tablet press with a pressure of 5 MPa, and each tablet weighed 1050 mg. Break up the pressed large tablets, pass through a 10-mesh sieve, and pack in gelatin capsules, each capsule is loaded with 350 mg of medicine. Under the condition of pH 6.0, the dissolution rate was measured by basket method at 100 rpm, and the selected time points were 5, 10, 15, 20, 30, 45 and...
Embodiment 3
[0064] Preparation of solid dispersion of palbociclib and PVP-K30 after mixing with acidic materials
[0065] Take by weighing 100 mg of palbociclib bulk drug, 200 mg of tartaric acid, put into a 10 ml vial, add 2 ml of purified water, and mix and dissolve. Add 200 mg of PVP-K30 (product of BASF, Germany, full name: povidone K30) to the above solution, and dissolve it by ultrasonic and hand shaking. In the fume hood, stir under heating conditions to help water evaporate; assist with nitrogen gas to help water evaporate. When the inside of the vial is gelatinous and there is no liquid loss, stop heating and stirring. Put it in a vacuum desiccator overnight at 40°C. It was taken out the next day and a solid dispersion was made.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com