Conjugate small molecule material based on pyridine pyrrolidone and diindene dithiophene condensed ring and preparation method of condensed ring
A pyridinepyrrole diketone and dithiophene technology, which can be used in semiconductor/solid-state device manufacturing, photovoltaic power generation, electrical components, etc., and can solve problems such as poor film-forming performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1: Synthesis of small molecule material M4
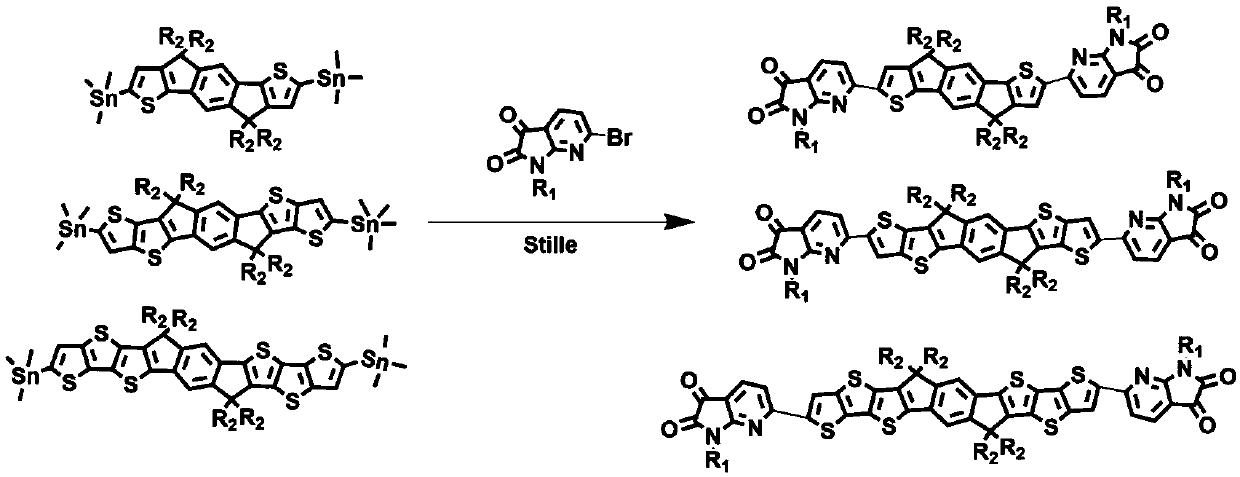
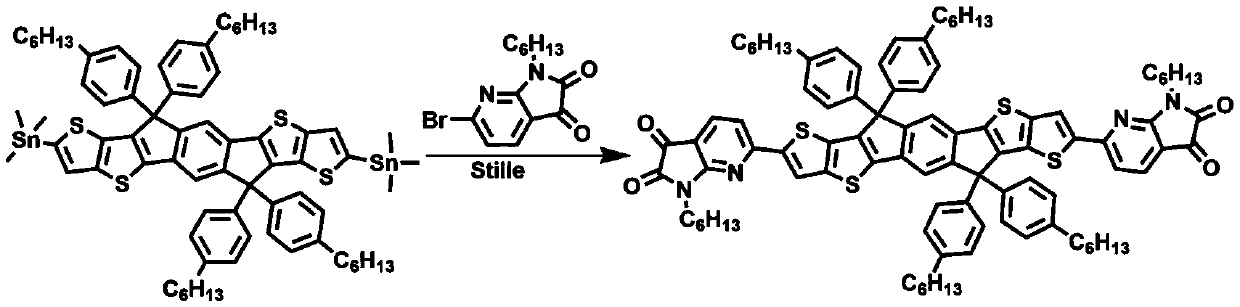
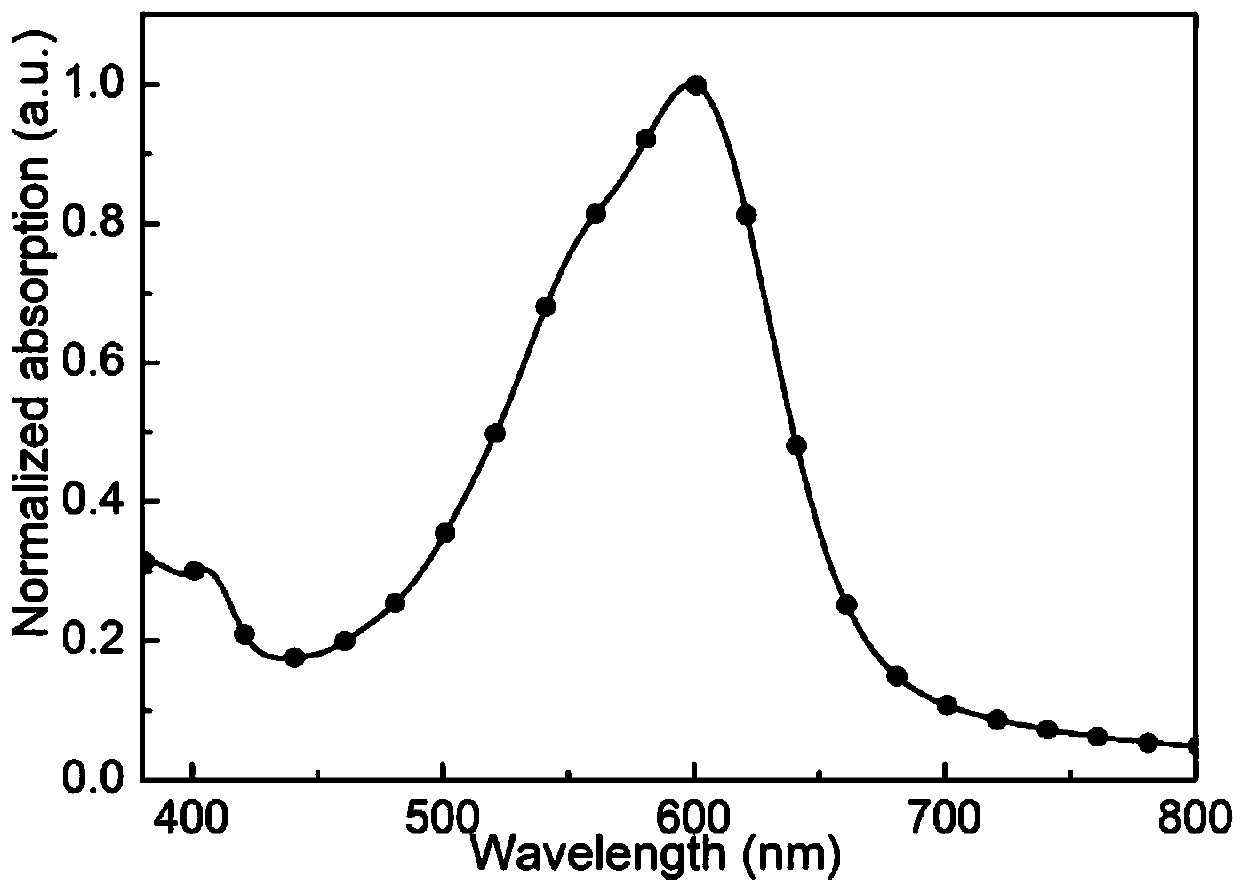
[0021] The physical diagram of the synthesis path of the small molecule material M4 is as follows figure 2 As shown, the specific steps are: in a 100mL reaction flask, add 1.6mmol of tin monomer of diindenodithiophene fused ring, 2.3 times of brominated monomer of diketopyridinepyrrole, add anhydrous toluene (also can be anhydrous water (tetrahydrofuran, chlorobenzene) 20mL, replaced under nitrogen atmosphere for 30 minutes, added 2% catalyst (Pd 2 dba 3 ) and 8% ligand (P(o-toly) 3 ), reacted at 105°C for 18 hours, cooled the reactant to room temperature, added 50mL of water and 30mL of dichloromethane for extraction, extracted three times, collected the organic layer, dried over anhydrous magnesium sulfate, then suction filtered, rotary evaporated the organic solvent, and finally used petroleum Ether: diethyl ether: dichloromethane (5:1:1) to purify the product to obtain the small purple molecule M4 (120mg, 5...
Embodiment 2
[0025] Using other monomers to synthesize other series of small molecule materials, the specific steps are the same as in Example 1: add 1.6mmol of tin monomer of bisindenodithiophene condensed ring in a 100mL glass reaction bottle, and 2.3 times the bromination of diketopyridinepyrrole Monomer, add 20 mL of anhydrous toluene (also can be anhydrous tetrahydrofuran, chlorobenzene), replace under nitrogen atmosphere for 30 minutes, add 2% catalyst (Pd 2 dba 3 ) and 8% ligand (P(o-toly) 3 ), reacted at 105°C for 18 hours, cooled the reactant to room temperature, added 50mL of water and 30mL of dichloromethane for extraction, extracted three times, collected the organic layer, dried over anhydrous magnesium sulfate, then suction filtered, rotary evaporated the organic solvent, and finally used petroleum Ether: diethyl ether: dichloromethane (5:1:1) to purify the product to obtain a small purple molecule whose structural formula is shown in Table 1.
[0026] The structural formul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com