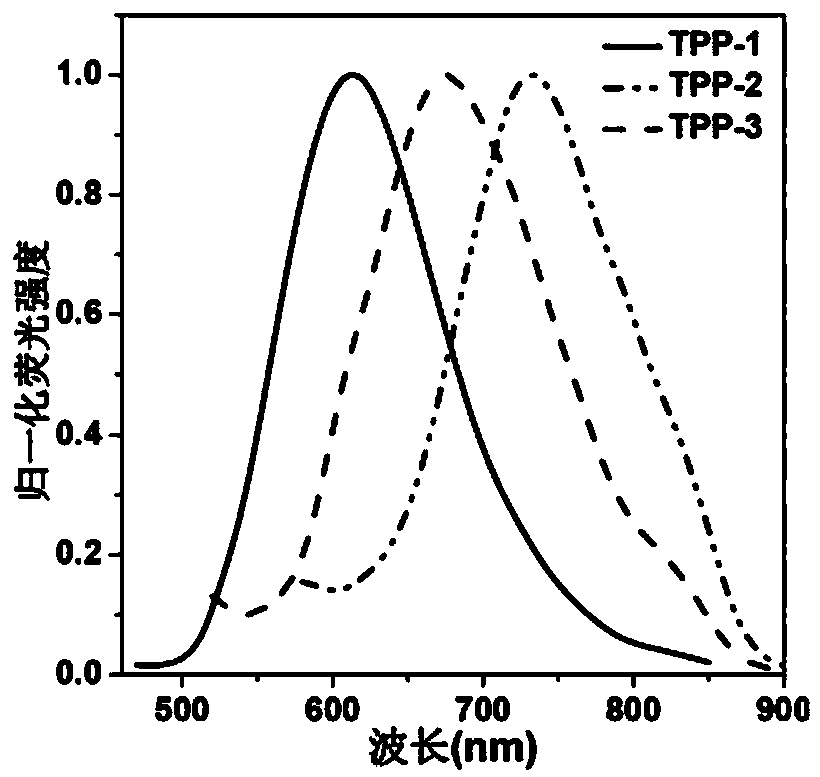
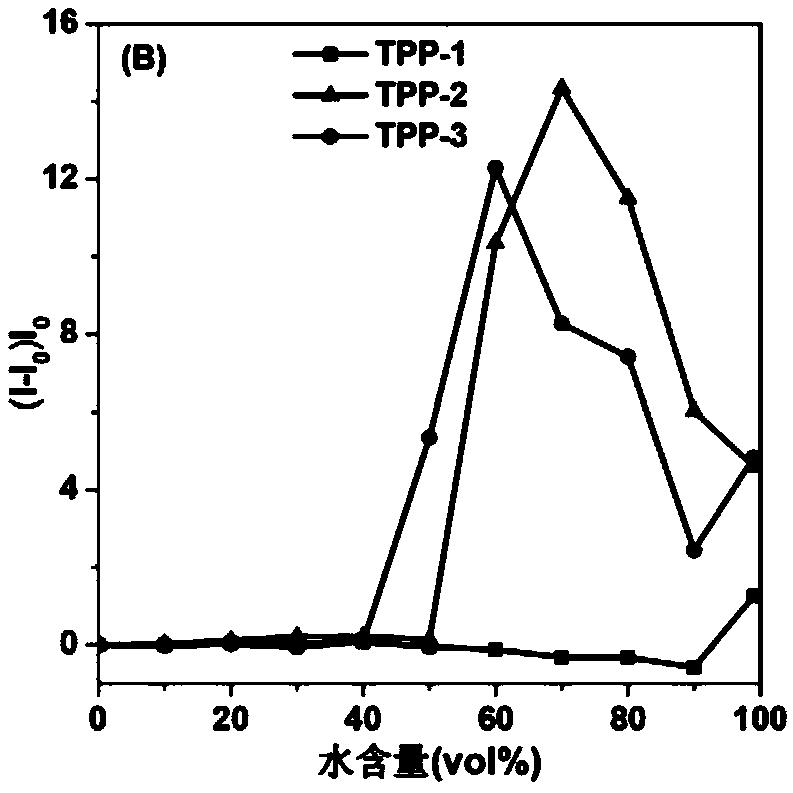
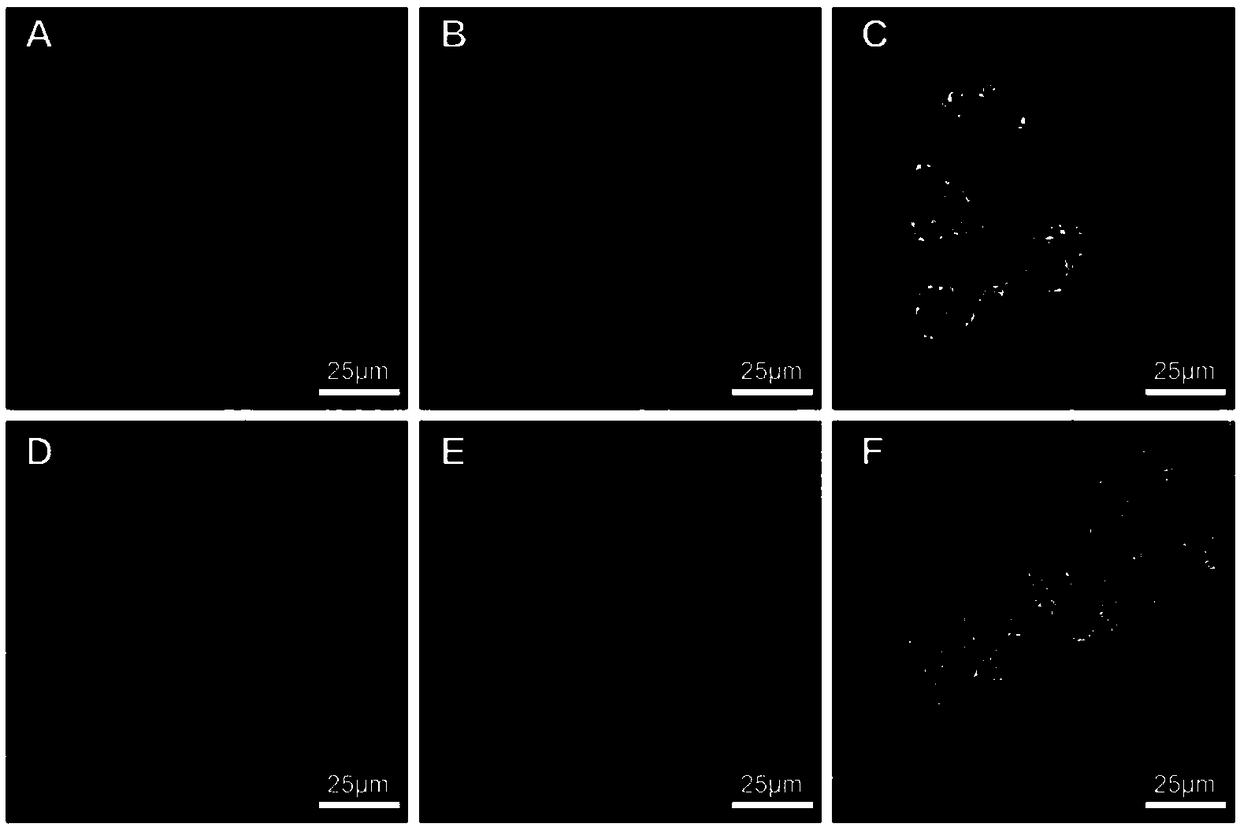
Near-infrared fluorescent compound with AIE performance as well as preparation method and application thereof
A fluorescent compound and near-infrared technology, applied in chemical instruments and methods, organic chemistry, fluorescence/phosphorescence, etc., can solve the problems of real-time imaging of mitochondria, no washing and photostability, etc., and achieve great application value and prospects, good Biocompatibility, photodamage reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0059] In a preferred embodiment of the present invention, the preparation method includes the following steps:
[0060] Compound A and indole salt are refluxed and reacted in a solvent to obtain compound B, and an aqueous solution of hexafluorophosphate is added and stirred for reaction to obtain the fluorescent compound.
[0061] In a preferred embodiment of the present invention, the solvent includes one or both of ethanol and methanol.
[0062] In a preferred embodiment of the present invention, the preparation method includes the following steps:
[0063] Compound A reacts with indole salt in ethanol and / or methanol under reflux for 12-30h, removes ethanol and / or methanol, dissolves in tetrahydrofuran; adds saturated hexafluorophosphate aqueous solution, stirs at room temperature for 0.2-1h, removes solvent , and purify to obtain the fluorescent compound. The normal temperature refers to 25±5°C.
[0064] In a preferred embodiment of the present invention, the hexafluor...
Embodiment 1
[0096] This embodiment provides three preparation methods of compound A, the steps are as follows:
[0097] 1. TPP-CHO-1 preparation of
[0098] (1) Add 5.02mmol of 1,2-diphenylethyne, 20.08mmol of p-aminobenzoic acid ethyl ester and 0.5mmol of CuCl into a 100mL polymerization tube, vacuumize and fill with nitrogen three times, under nitrogen protection conditions, React at 120°C for 24 hours; after the reaction, dissolve with dichloromethane, wash three times with water, wash three times with 5% hydrochloric acid aqueous solution, dry with anhydrous magnesium sulfate, remove the solvent to obtain the crude product; pass the crude product through volume The eluent of methylene chloride and petroleum ether at a ratio of 1:2 is separated and purified to obtain 1-ethyl p-benzoate-2,5-diphenylpyrrole;
[0099] (2) At 0°C, 270 μL of POCl 3 Slowly add dropwise to 24mL of DMF, stir at room temperature for 1h, add 2.0mmol of 1-p-benzoic acid ethyl ester-2,5-diphenylpyrrole to the ...
Embodiment 2
[0119] This example provides TPP-1 The preparation method, the steps are as follows:
[0120] Take 0.6mmol of TPP-CHO-1 prepared in Example 1 and 0.65mmol of 1-ethyl-2,3,3-trimethyl-3H-indol-1-ium in 15mL of absolute ethanol Reflux for 24 hours; after the reaction, cool to room temperature, remove the solvent, collect the solid, dissolve the solid in 5 mL of THF, and then add 5 mL of saturated KPF 6 After stirring for 30 min, the solvent was removed and purified by silica gel column chromatography, the eluent was dichloromethane and methanol with a volume ratio of 10:1 to obtain TPP-1.
[0121] TPP-1 was characterized by nuclear magnetic resonance spectrometer and mass spectrometer, and the data are as follows:
[0122] 1 H NMR (400MHz, DMSO-d 6 )δ (ppm): 7.99-7.85 (m, 3H), 7.86-7.75 (m, 2H), 7.68 (s, 1H), 7.63-7.50 (m, 2H), 7.50-7.41 (m, 4H), 7.33 -7.26(m,7H),7.20(d,J=6.9Hz,2H),4.58(q,J=7.3Hz,2H),4.28(q,J=8.9,6.8Hz,2H),1.53(s, 6H), 1.42(t, J=7.3Hz, 3H), 1.29(t, J=6.8Hz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com