Preparation method of 2,6-dihydroxybenzoic acid
A technology of dihydroxybenzoic acid and dichlorobenzaldehyde, applied in the chemical industry, can solve the problems of high raw material prices, cumbersome operation steps, and difficult treatment of phenol-containing strong acid wastewater, and achieve the effect of cheap raw materials, simple operation, and easy production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
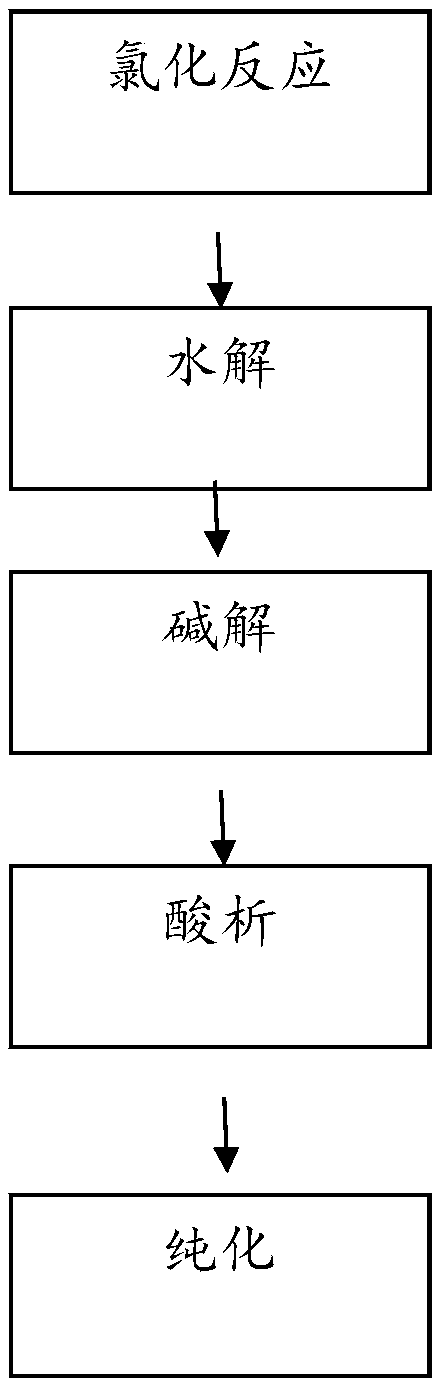
example 1
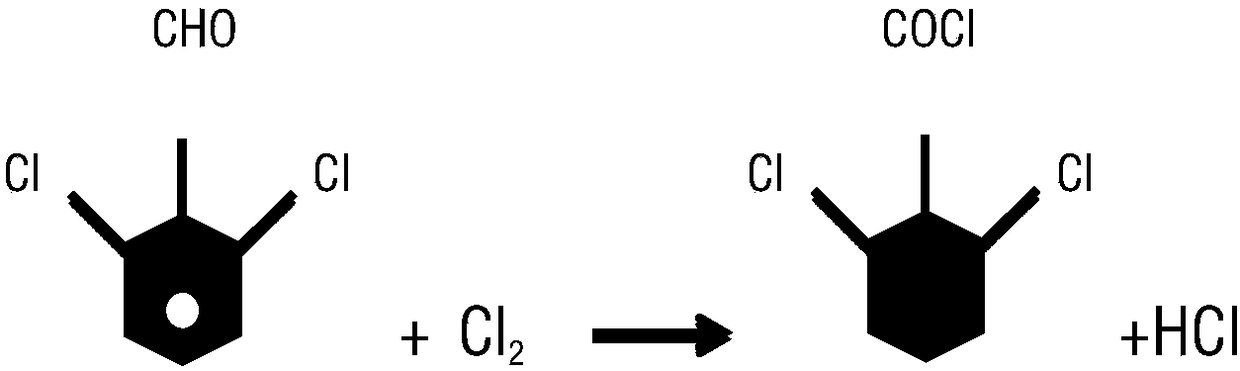
[0050] 1) Chlorination: Put 1L of organic solvent and 350g of 2,6-dichlorobenzaldehyde into the reaction kettle, and pass chlorine gas under the condition of temperature control at 50-150°C for chlorination. After passing the test, stop the chlorine flow, and the chlorination is over .
[0051] 2) Rectification: Put the chlorinated liquid into the rectification kettle, heat up the rectification and separate it, first distill out the solvent for recycling, and when the temperature at the top of the tower reaches 140-143°C, distill out qualified 2,6-dichlorobenzoyl chloride, 393.6 g of 2,6-dichlorobenzoyl chloride was weighed, and the conversion rate was 93.9%.
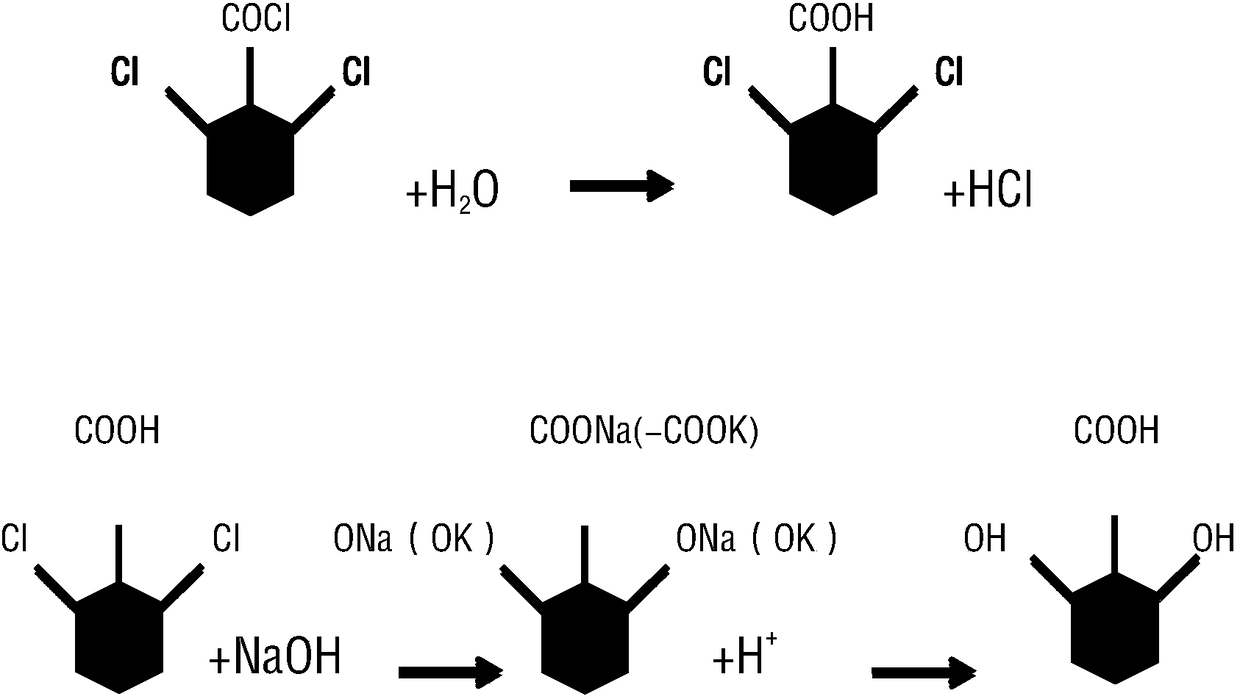
[0052] 3) Hydrolysis: Slowly add 1L of water to 2,6-dichlorobenzoyl chloride, slowly raise the temperature to reflux, cool down and filter to obtain 357.4 g of hydrolyzed product 2,6-dichlorobenzoic acid, with a conversion rate of 99.6%.
[0053] 4) Alkaline hydrolysis: Put the 2,6-dichlorobenzoic acid-base solution in...
example 2
[0056] 1) Chlorination: 1.5L of organic solvent and 525g of 2,6-dichlorobenzaldehyde were put into the reaction kettle, and chlorine gas was introduced into the reactor at a temperature of 50-150°C for chlorination. Finish.
[0057] 2) Rectification: Put the chlorinated liquid into the rectification kettle, heat up the rectification and separate it, first distill out the solvent for recycling, and when the temperature at the top of the tower reaches 140-143°C, distill out qualified 2,6-dichlorobenzoyl chloride, 586.6 g of 2,6-dichlorobenzoyl chloride was weighed, and the conversion rate was 93.7%.
[0058] 3) Hydrolysis: 1.5 L of water was slowly added to 2,6-dichlorobenzoyl chloride, and the temperature was raised slowly to reflux, and the temperature was lowered and filtered to obtain 532.7 g of hydrolyzed product 2,6-dichlorobenzoic acid, with a conversion rate of 99.6%.
[0059] 4) Alkaline hydrolysis: Put the 2,6-dichlorobenzoic acid-base solution into the reaction kettl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com