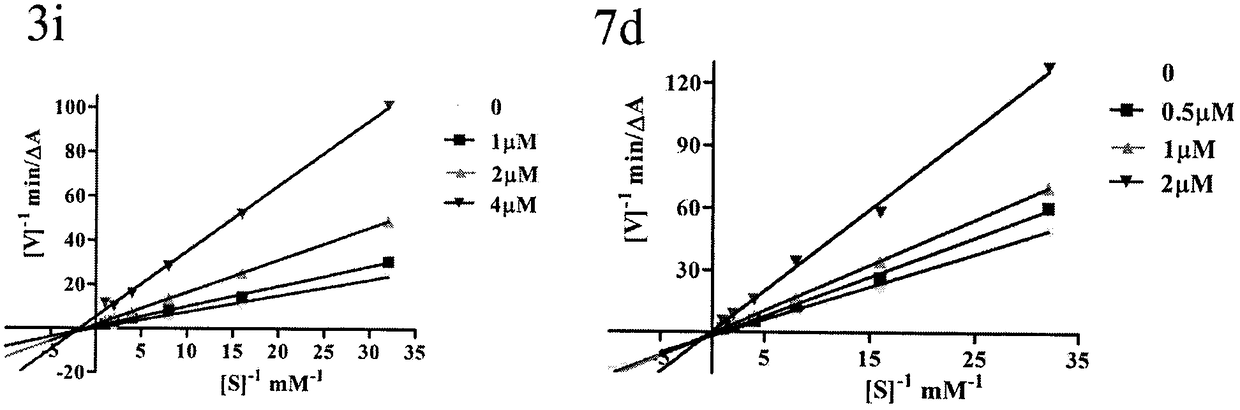
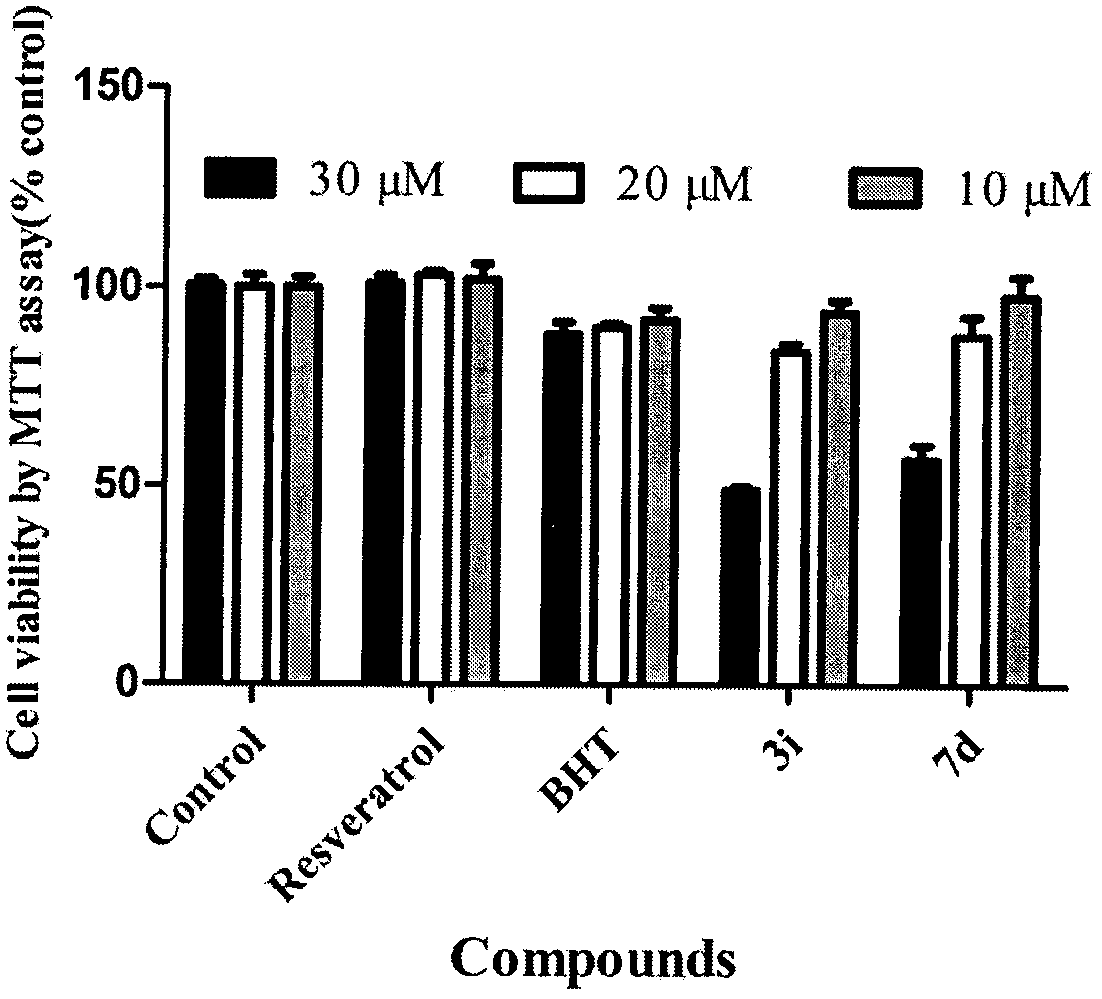
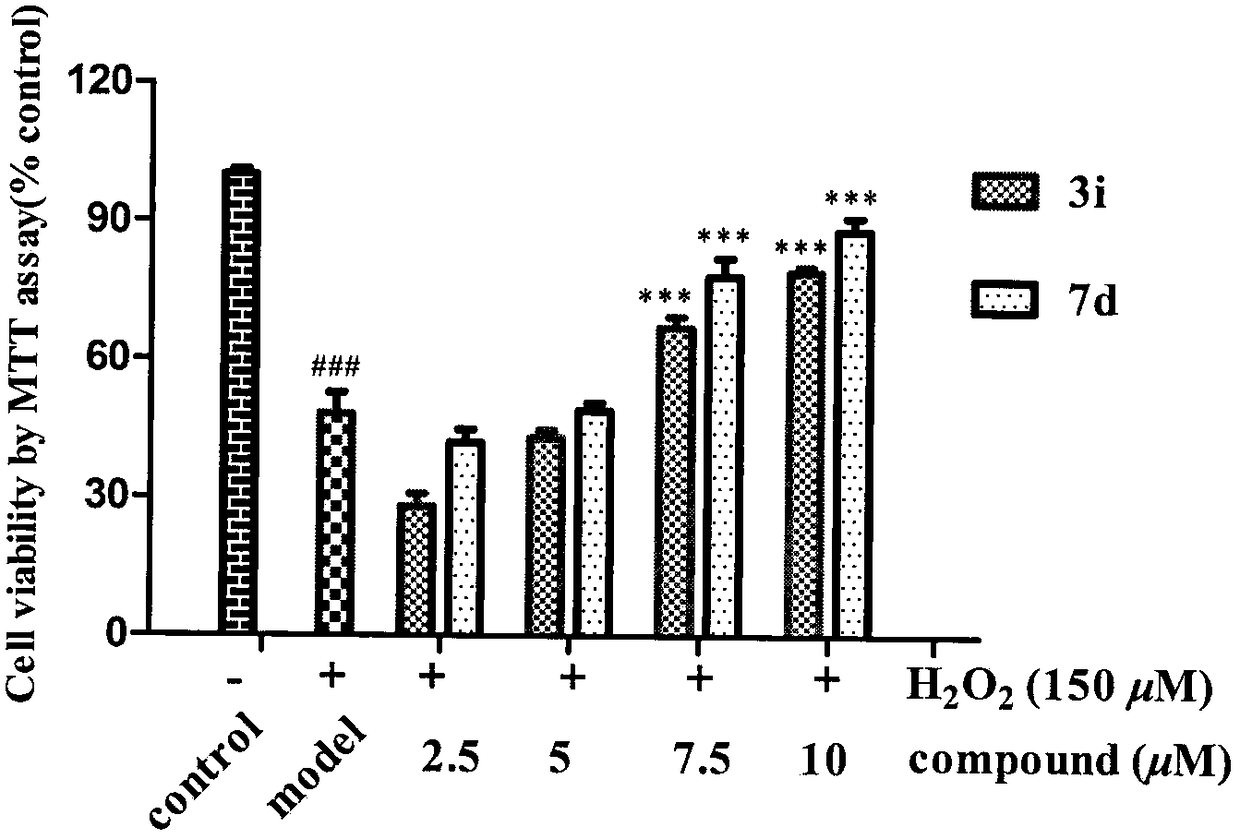
Donepezil-BHT heterozygotes and preparation method and application thereof in treatment of Alzheimer's disease
A technology of donepezil and -BHT, which is applied in the field of donepezil-BHT hybrid, its preparation and its use in the treatment of Alzheimer's disease, and can solve the problems of inhibiting the pathological process of Alzheimer's disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Preparation of 4-(N-1-benzylpiperidine)amino-2,4-di-tert-butyl-p-hydroxybenzamide (3a).
[0077] Dissolve 100mg (0.39mmol, 1eq) of the raw material 2,4-di-tert-butyl p-hydroxybenzoic acid (1a) in 6ml of anhydrous dichloromethane, and add condensing agent 1-hydroxybenzotriazole (HOBT) 49.74mg (0.36 mmol, 1.1eq) and 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDCI) 69.62mg (0.36mmol, 1.1eq), drop triethylamine 151μl (1.09 mmol, 3eq), after stirring and reacting under ice bath for 1 hour, add 4-amino-1-benzylpiperidine (2a) 69.1mg (0.36mmol, 1eq) dropwise in dichloromethane solution to react overnight, monitor the reaction until the amine is complete , washed three times with saturated sodium bicarbonate, washed three times with saturated sodium chloride until HOBT was cleaned, the organic phase was combined and mixed and passed through a silica gel column, and the dichloromethane / methanol product system was obtained to obtain about 140 mg of the target pro...
Embodiment 2
[0079] Preparation of 4-(N-1-benzylpiperidine)aminomethyl-2,4-di-tert-butyl-p-hydroxybenzamide (3b)
[0080] The preparation process is the same as 1, but the raw material 4-amino-1-benzylpiperidine (2a) is replaced with 4-aminomethyl-1-benzylpiperidine (2b), the yield is 82%; white solid, m.p.217-218 °C, 1 H NMR (500MHz, DMSO) δ8.27(d, J=7.7Hz, 1H), 7.60(s, 2H), 7.31(m, 5H), 3.45(s, 2H), 3.13(t, J=6.2Hz , 2H), 2.81(d, J=11.0Hz, 2H), 1.90(t, J=11.0Hz, 2H), 1.64(d, J=12.2Hz, 2H), 1.54(d, J=6.2Hz, 1H ), 1.41(s, 18H), 1.22-1.11(m, 2H); 13 C NMR (126MHz, DMSO) δ167.51, 156.51, 139.24, 138.64, 129.20, 128.56, 127.24, 126.45, 124.43, 62.91, 53.46, 45.20, 36.33, 35.03, 30.71, 30.36. ESI-MS m / z [: 437 M+H] + ;HRMS(ESI)m / z: 437.3164[M+H] + (calcd for 437.3163, C 28 h 41 N 2 o 2 ).
Embodiment 3
[0082] Preparation of 4-(N-1-benzylpiperidine)aminoethyl-2,4-di-tert-butyl p-hydroxybenzamide (3c)
[0083] The preparation process is the same as 1, the raw material 4-amino-1-benzylpiperidine (2a) is replaced with 4-aminoethyl-1-benzylpiperidine (2c), the yield is 75%; white solid, m.p.182-183 °C, 1 H NMR (500MHz, CDCl3) δ7.61(s, 2H), 7.42-7.33(m, 4H), 7.32(s, 1H), 5.99(s, 1H), 5.57(s, 1H), 3.60(s, 2H), 3.51(dd, J=14.1, 6.4Hz, 2H), 2.98(d, J=10.3Hz, 2H), 2.06(s, 2H), 1.78(d, J=10.3Hz, 2H), 1.61( d, J=6.4Hz, 2H), 1.50(s, 18H), 1.43(s, 2H), 1.32(d, J=14.1Hz, 1H); 13 C NMR (126MHz, DMSO) δ167.95, 156.51, 135.96, 129.56, 128.31, 126.04, 123.98, 53.30, 37.76, 36.50, 34.43, 33.62, 31.54, 30.20. ESI-MS m / z: 451.3 [M+H] + ;HRMS(ESI)m / z: 451.3317[M+H] + (calcd for 451.3319, C 29 h 43 N 2 o 2 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com