Preparation method of dinitroglycol guanidine
A technology of dinitroglycoguanidine and guanidine nitrate, which is applied in the direction of organic chemistry, can solve problems such as side reactions, and achieve the effects of increased yield, good economic benefits, and less impurity content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
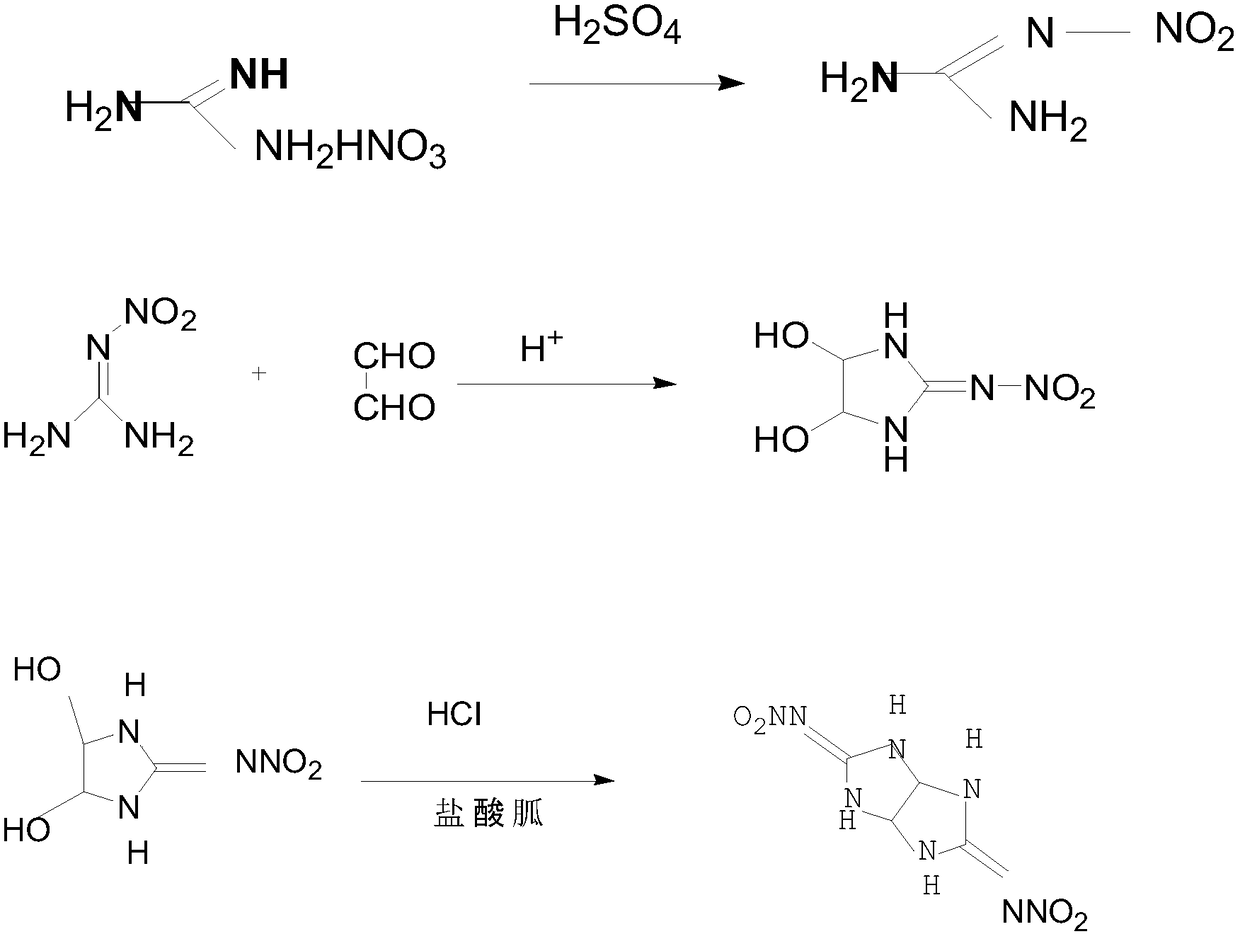
[0024] This embodiment discloses a preparation method of dinitroglycoguanidine. The preparation method adopts ultrasonic and low-temperature dropwise addition. The specific steps of the preparation method are as follows:
[0025] Add 200g of concentrated sulfuric acid into a 500ml round bottom flask, the concentration of sulfuric acid is 98%, start stirring and slowly add 100g of guanidine nitrate, the feeding speed is controlled at 30min, and the feeding temperature does not exceed 30°C. After the addition of guanidine nitrate, slowly heat up to 40 ℃, heat preservation reaction for 0.5h, the material was added to 800g of ice water, rapidly stirred to cool down to 25 ℃, then filtered and washed with water to obtain nitroguanidine.
[0026] Add 174g of 40% glyoxal aqueous solution into a 500ml round-bottom flask, start stirring, slowly add 130g of nitroguanidine, and drop 52g of 35% hydrochloric acid at the same time, transfer the device to an ultrasonic system after the additio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com