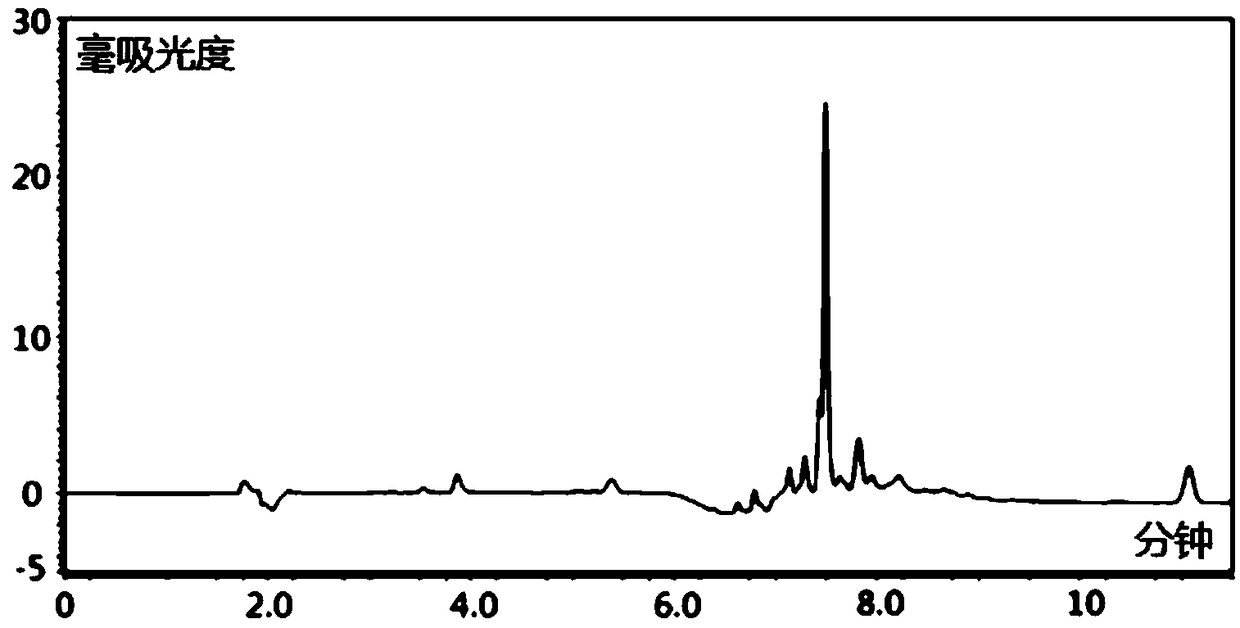
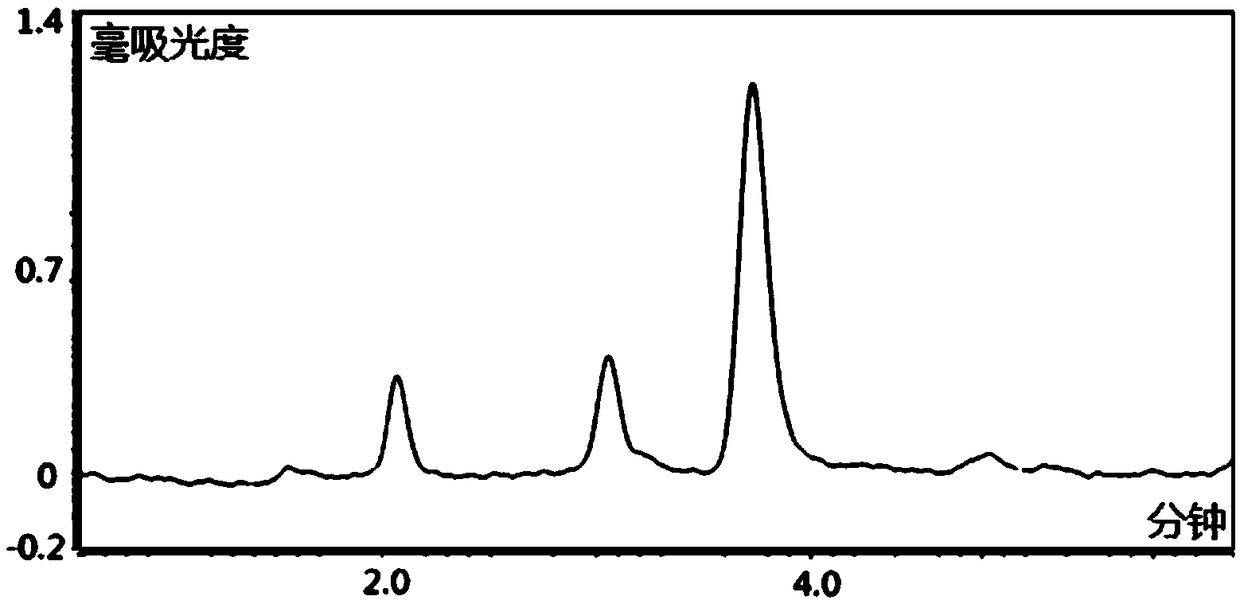
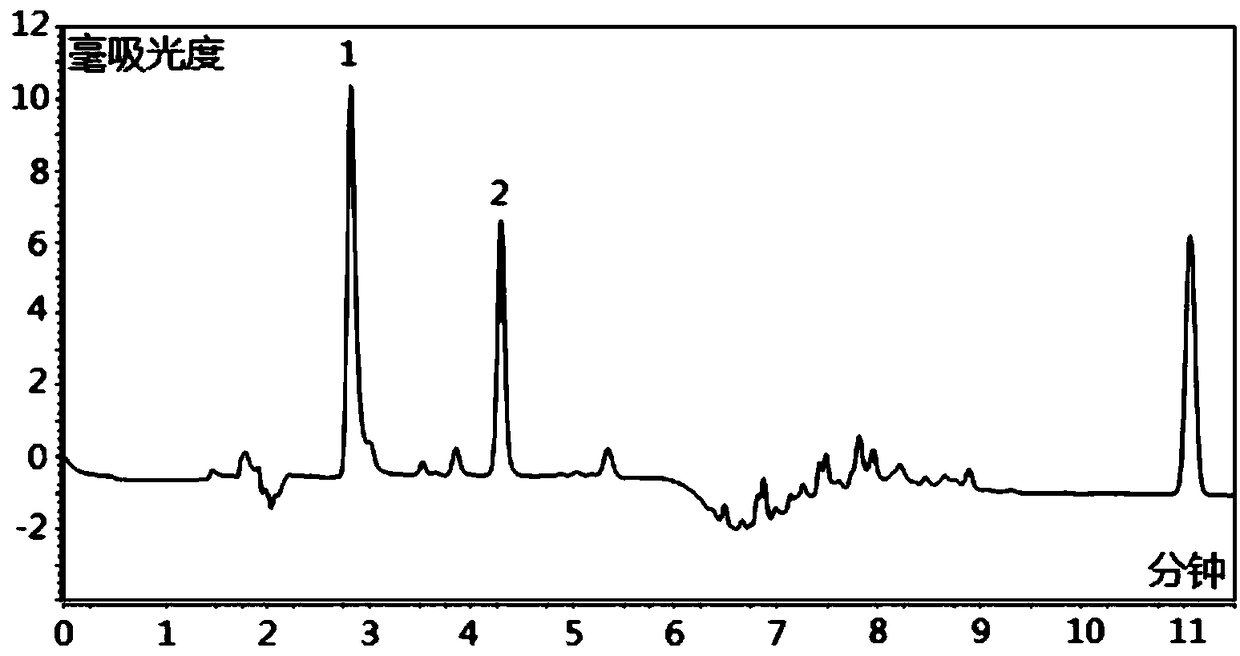
HPLC-DAD method for determinating quetiapine drug concentration of human serum
A technology of drug concentration and quetiapine, which is applied in the field of clinical blood drug concentration monitoring of psychiatric drugs, can solve the problems of complex preprocessing, inaccurate results, and narrow quantitative analysis range, and achieves the effect of reducing reagent pollution.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] (1) Preparation of standard working solution
[0043] (1) Preparation of standard working solution:
[0044] Accurately weigh 5.57mg of quetiapine fumarate standard substance (equivalent to 4.83mg of quetiapine mass) in a 15mL centrifuge tube, dissolve with methanol, and prepare stock solution S1 with a quetiapine concentration of 1.0mg / mL. Dilute the standard stock solution S1 with methanol to prepare quetiapine series standard working solutions containing 1.0, 2.0, 3.0, 6.0, 10, 20, and 60 μg / mL concentrations respectively, and store them in a -80°C refrigerator;
[0045] (2) Preparation of internal standard working solution
[0046] Accurately weigh 2.31 mg of genistein standard substance (equivalent to 2.30 mg of genistein) in a 5mL centrifuge tube, dissolve in methanol, and make a stock solution ISS1 with a concentration of 1.0 mg / mL, and use the standard stock solution ISS1 with Dilute with methanol to obtain an internal standard working solution with a concentr...
Embodiment 2
[0056] (1) Preparation of standard working solution
[0057] (1) Preparation of standard working solution:
[0058] Accurately weigh 5.57mg of quetiapine fumarate standard substance (equivalent to 4.83mg of quetiapine mass) in a 15mL centrifuge tube, dissolve with methanol, and prepare stock solution S1 with a quetiapine concentration of 1.0mg / mL. Dilute the standard stock solution S1 with methanol to prepare quetiapine series standard working solutions containing 1.0, 2.0, 3.0, 6.0, 10, 20, and 60 μg / mL concentrations respectively, and store them in a -80°C refrigerator;
[0059] (2) Preparation of internal standard working solution
[0060] Accurately weigh 2.31 mg of genistein standard substance (equivalent to 2.30 mg of genistein) in a 5mL centrifuge tube, dissolve in methanol, and make a stock solution ISS1 with a concentration of 1.0 mg / mL, and use the standard stock solution ISS1 with Dilute with methanol to obtain an internal standard working solution with a concentr...
Embodiment 3
[0070] (1) Preparation of standard working solution
[0071] (1) Preparation of standard working solution:
[0072] Accurately weigh 5.57mg of quetiapine fumarate standard substance (equivalent to 4.83mg of quetiapine mass) in a 15mL centrifuge tube, dissolve with methanol, and prepare stock solution S1 with a quetiapine concentration of 1.0mg / mL. Dilute the standard stock solution S1 with methanol to prepare quetiapine series standard working solutions containing 1.0, 2.0, 3.0, 6.0, 10, 20, and 60 μg / mL concentrations respectively, and store them in a -80°C refrigerator;
[0073] (2) Preparation of internal standard working solution
[0074] Accurately weigh 2.31 mg of genistein standard substance (equivalent to 2.30 mg of genistein) in a 5mL centrifuge tube, dissolve in methanol, and make a stock solution ISS1 with a concentration of 1.0 mg / mL, and use the standard stock solution ISS1 with Dilute with methanol to obtain an internal standard working solution with a concentr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com