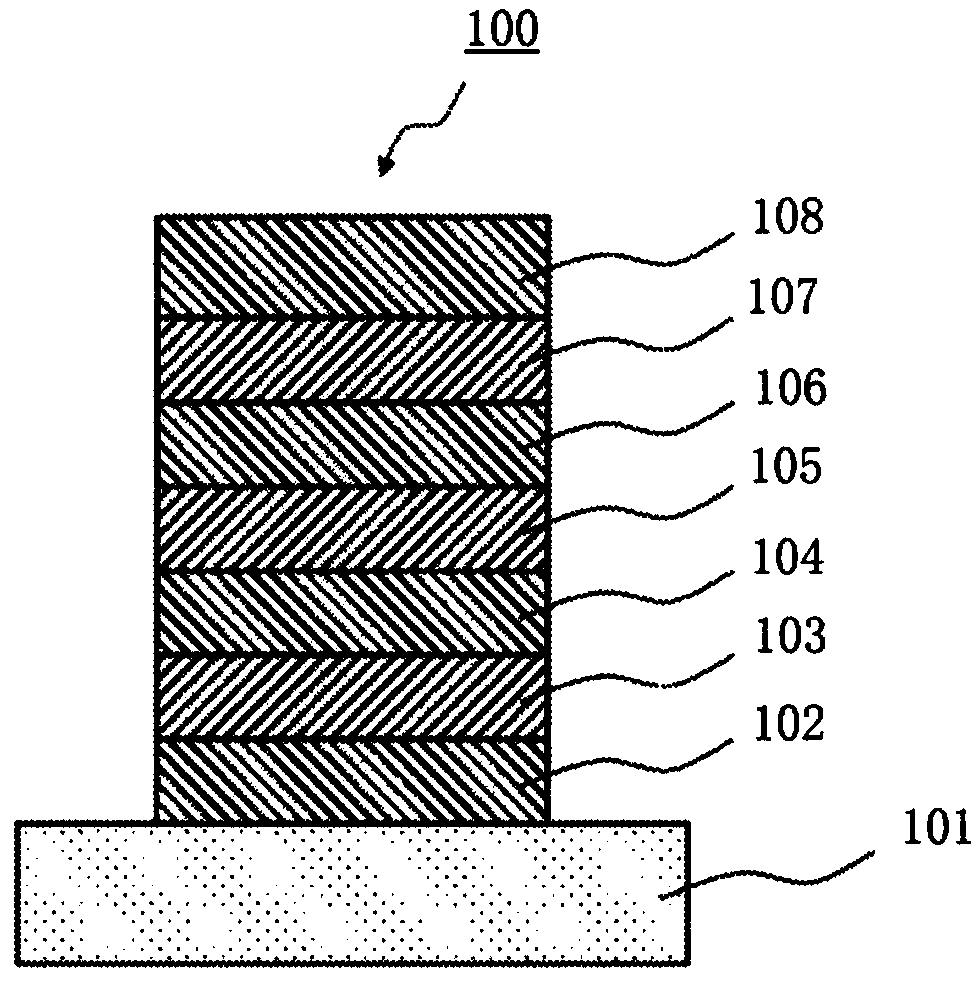
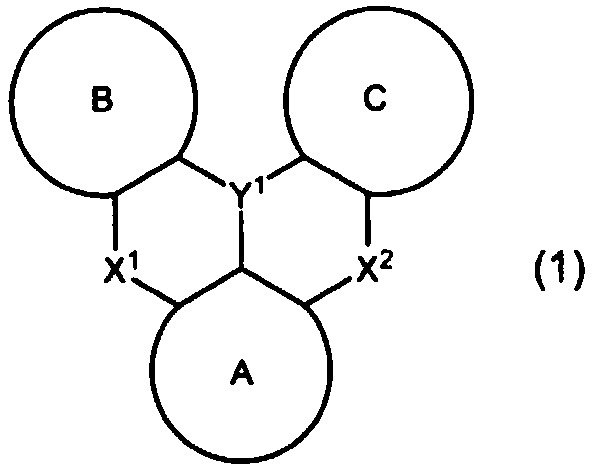
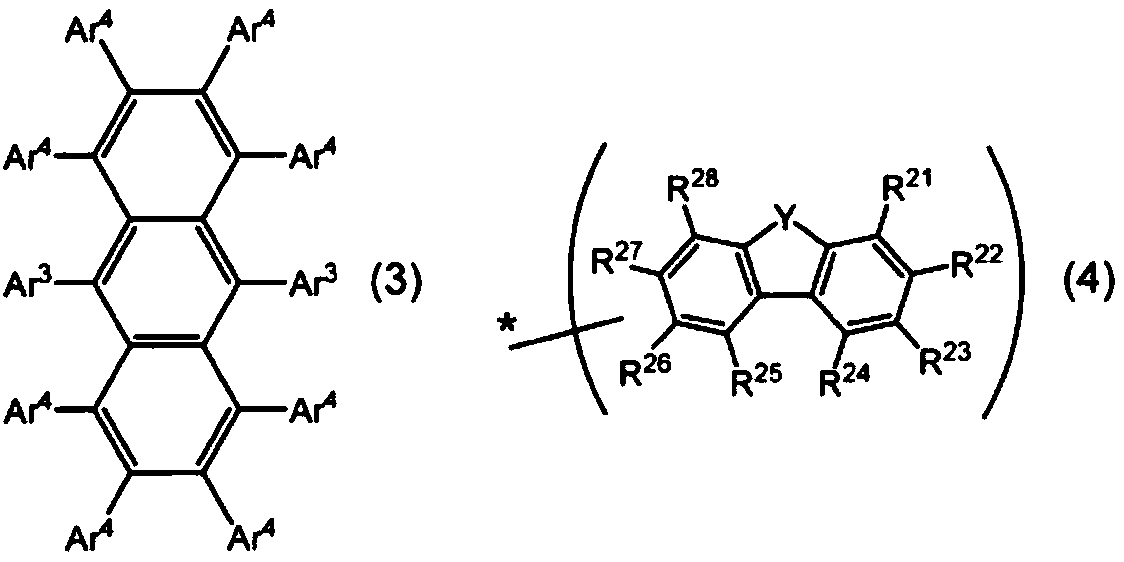
Organic electroluminescent element
A technology of light-emitting element and organic electric field, applied in the fields of electrical components, light-emitting materials, organic chemistry, etc., which can solve the problems of different electronic states, unknown characteristics, and undocumented methods for producing NO-linked compounds.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0561] Hereinafter, although an Example demonstrates this invention more concretely, this invention is not limited to these Examples. First, synthesis examples of polycyclic aromatic compounds and polymers thereof will be described below.
Synthetic example (1
[0563] Compound (1-1152): 9-([1,1'-biphenyl]-4-yl)-5,12-diphenyl-5,9-dihydro-5,9-diaza-13b- Synthesis of boranaphtho[3,2,1-de]anthracene
[0564] [chem 99]
[0565]
[0566] Under nitrogen atmosphere, and at 80 ℃, put diphenylamine (37.5g), 1-bromo-2,3-dichlorobenzene (50.0g), Pd-132 (Johnson Matthey (Johnson Matthey) ))(0.8g), NaOtBu (32.0g) and xylene (500ml) were heated and stirred in a flask for 4 hours, then heated up to 120° C., and heated and stirred for 3 hours. After cooling the reaction liquid to room temperature, water and ethyl acetate were added and liquid-separated. Then, purification was carried out by silica gel column chromatography (developing solution: toluene / heptane=1 / 20 (volume ratio)) to obtain 2,3-dichloro-N,N-diphenylaniline (63.0 g) .
[0567] [chemical 100]
[0568]
[0569] Under a nitrogen atmosphere, and at 120°C, 2,3-dichloro-N,N-diphenylaniline (16.2g), bis([1,1'-biphenyl]-4-yl) A flask of amine (15.0 g), Pd-132 (Johnson Matthey) (0.3 ...
Synthetic example (2
[0578] Compound (1-422): 5,9,11,15-tetraphenyl-5,9,11,15-tetrahydro-5,9,11,15-tetraaza-19b,20b-diborinaphthalene Synthesis of [3,2,1-de:1',2',3'-jk]pentacene
[0579] [chem 103]
[0580]
[0581] Under a nitrogen atmosphere, 2,3-dichloro-N,N-diphenylaniline (36.0g), N 1 ,N 3 - A flask of diphenylbenzene-1,3-diamine (12.0 g), Pd-132 (Johnson Matthey) (0.3 g), NaOtBu (11.0 g) and xylene (150 ml) was heated and stirred for 3 hours. After cooling the reaction liquid to room temperature, water and ethyl acetate were added and liquid-separated. Then, purification was performed by silica gel column chromatography (developing solution: toluene / heptane mixed solvent). At this time, the ratio of toluene in the developing solution was gradually increased to elute the target substance. Further, it was purified by activated carbon column chromatography (developing solution: toluene), thereby obtaining N 1 ,N 1' -(1,3-phenylene)bis(2-chloro-N 1 ,N 3 ,N 3 -triphenylbenzene-1,3-d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electron work function | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com