Use of fam134b in the preparation of medicines for treating sepsis
A technology of drugs and reagents, applied in the fields of molecular biology and biomedicine, which can solve the problems of persistent infection, superinfection, immune dysfunction, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Example 1 Correlation between FAM134B and sepsis
[0071] 1. In vivo experiment
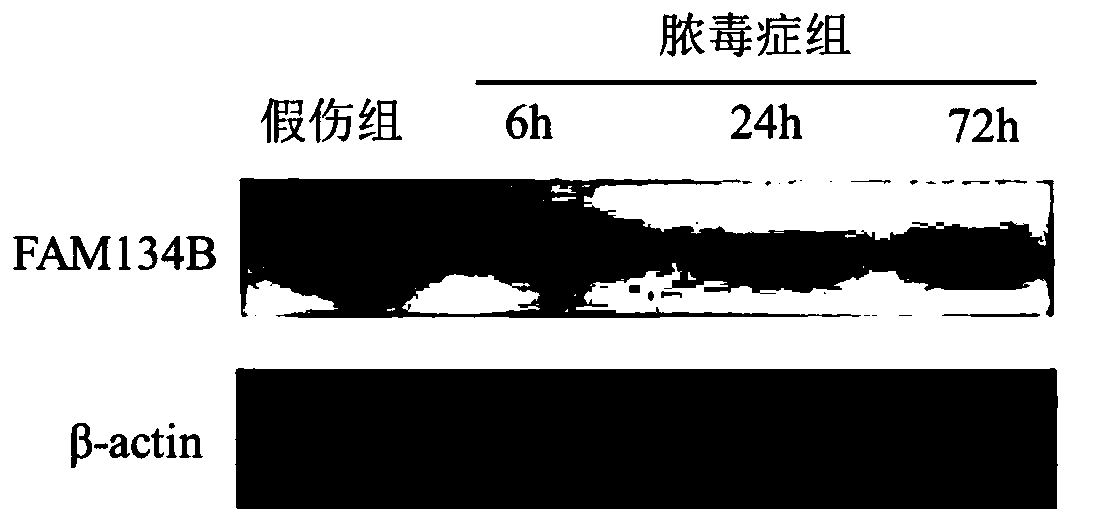
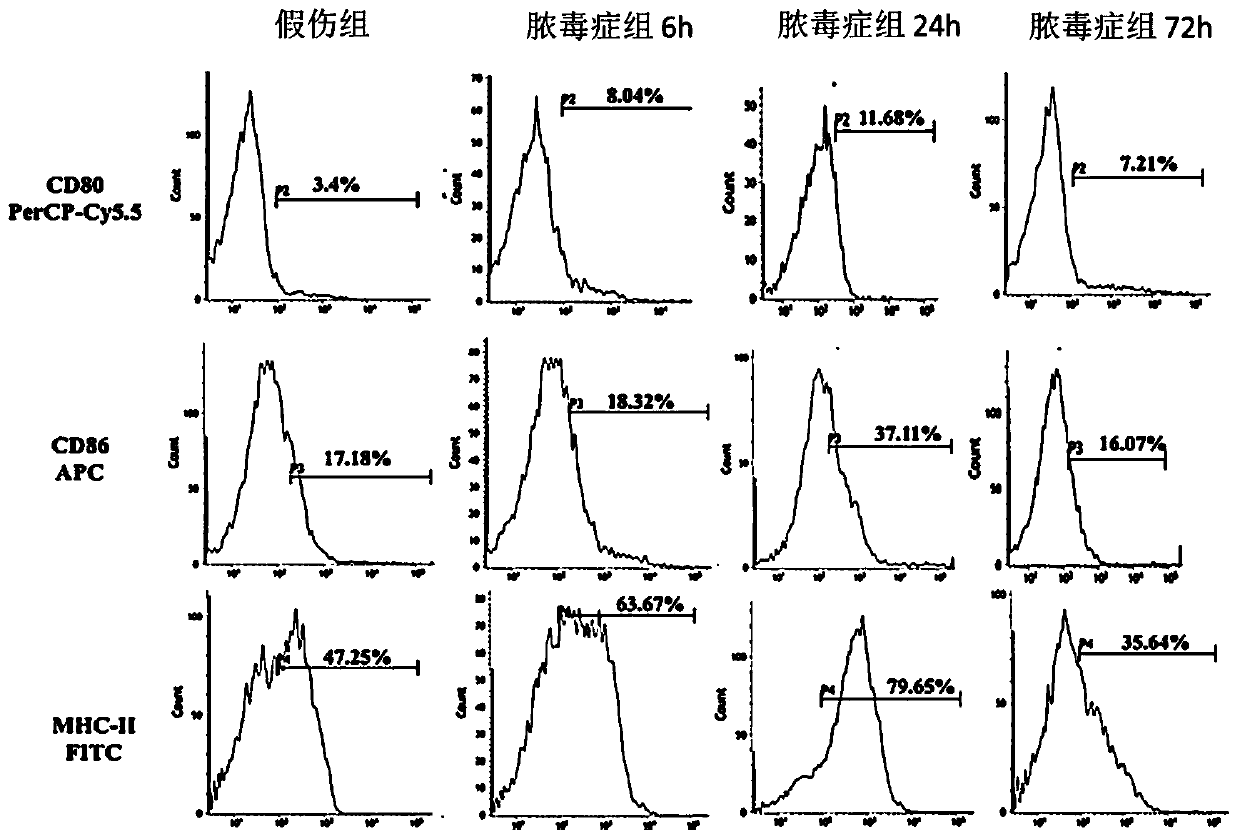
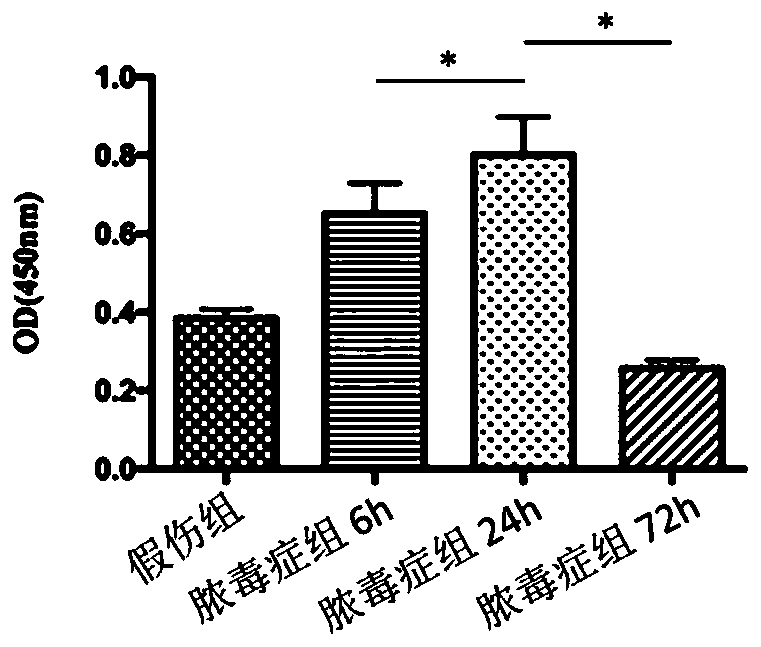
[0072] Take 6-8 weeks old wild-type male C57BL / 6 mice (body weight: 20-22g), carry out numbering, weigh, and divide into three groups randomly (random number method): normal group, sham injury group and sepsis group, The mice in the sepsis group were subjected to CLP to construct a sepsis model, and the mice in the sham injury group were opened to expose the cecum, and then the abdomen was closed after feeding. The animals were sacrificed at 6h, 24h, and 72h after the operation, and the spleens of the mice were removed. DCs were isolated and purified by immunomagnetic beads method (CD11c Microbeads), DC function was evaluated, and the expression of FAM134B in each group was detected and compared at different time points.
[0073] (1) Establishment of sepsis animal model
[0074] Male C57BL / 6 mice (20-22 g) aged 6-8 weeks were fasted for 12 hours before the operation and allowed to drink ...
Embodiment 2
[0093] Example 2 Effect of Interfering with FAM134B Gene Expression on DC Functional Activation
[0094] 1. In vivo experiment
[0095] 1.1 Obtaining of FAM134B knockout mice (FAM134B- / -)
[0096] Nanjing University-Nanjing Institute of Biomedicine is the earliest research institution in China that has passed AAALAC certification and has maintained the certification for the longest time. It is the only national genetic engineering mouse seed center certified by the Ministry of Science and Technology. The important model animals required for this application (FAM134B knockout mice, FAM134B- / -) have been produced and provided by Nanjing University-Nanjing Institute of Biomedicine:
[0097] a. Design and construction of sgRNA: design two sgRNAs between intron 3-4 and 3'-UTR position of Fam134b gene, and synthesize and construct them in vitro;
[0098]b. Microinjection: Inject the constructed sgRNA and Cas9 mRNA together into 0.5-day fertilized eggs, and transplant 20-25 injecte...
Embodiment 3
[0141] Example 3 Effect of Overexpression of FAM134B on DC Functional Activation
[0142] 1. Steps
[0143]The upstream and downstream sequences of the FAM134B gene were queried from Genbank, and specific amplification primers were designed using VectorNTI software. Introducing a restriction site, digesting the expression vector with a restriction endonuclease, obtaining the target gene, and preparing a linearized expression vector. The gene of interest was constructed into a linearized expression vector using T4 DNA ligase. Correct clones were verified by sequencing, and high-purity plasmid extraction was carried out. After the DC2.4 cells were transfected with the lentiviral vector containing overexpressed FAM134B for 9 hours, the medium of the cells was changed, and the DC2.4 cell line with stable genetic overexpression of FAM134B was obtained after continuing to culture for 72 hours. According to 2×10 6 DC2.4 cells were inoculated at the ratio of cells / ml and cultured ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com