Photoinduced shrinkage metal-organic framework compound
A metal-organic framework and photoshrinkage technology, which is applied in the field of photosensitive materials, achieves the effects of high volume control accuracy, mild reaction conditions, and fast photoconversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
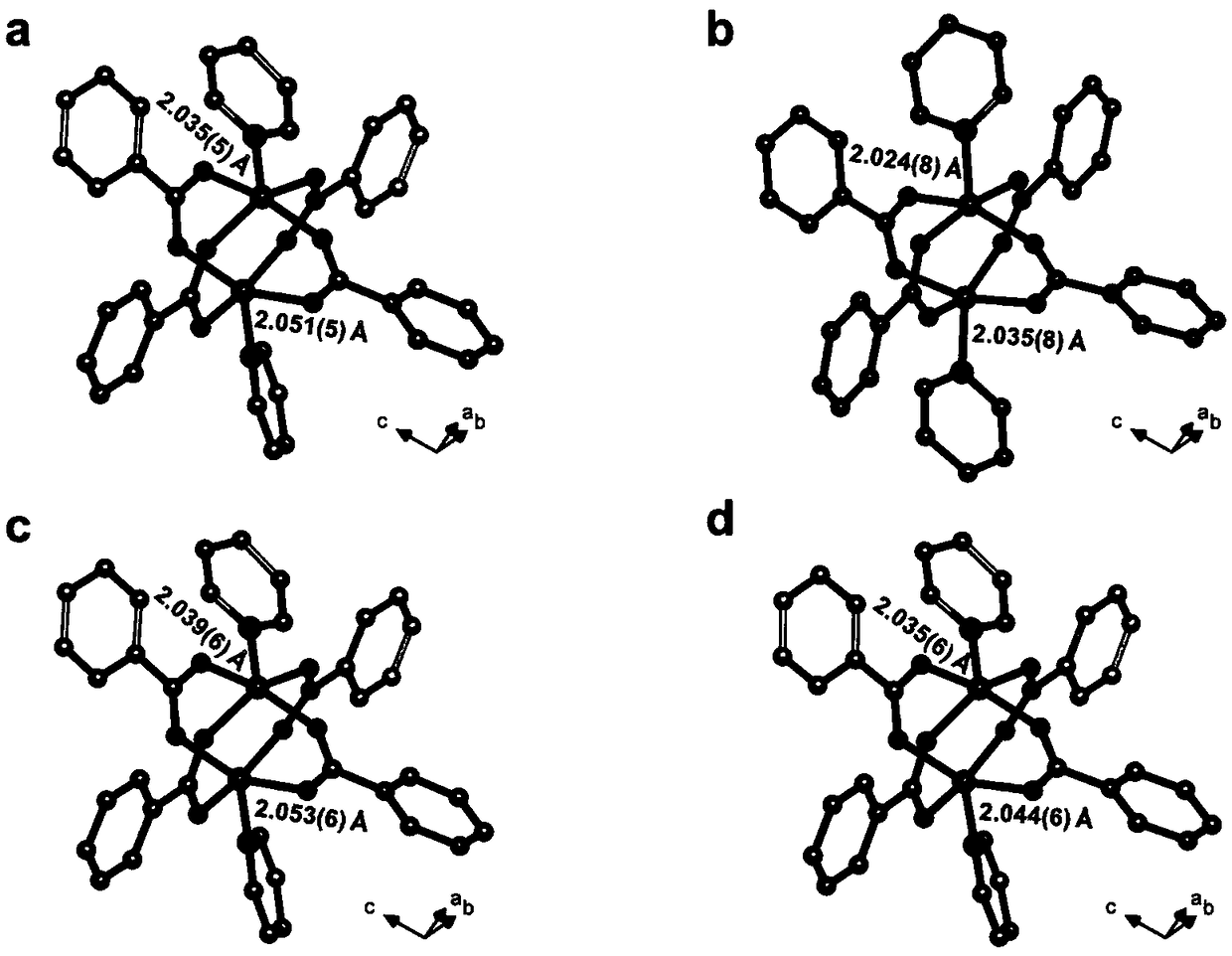
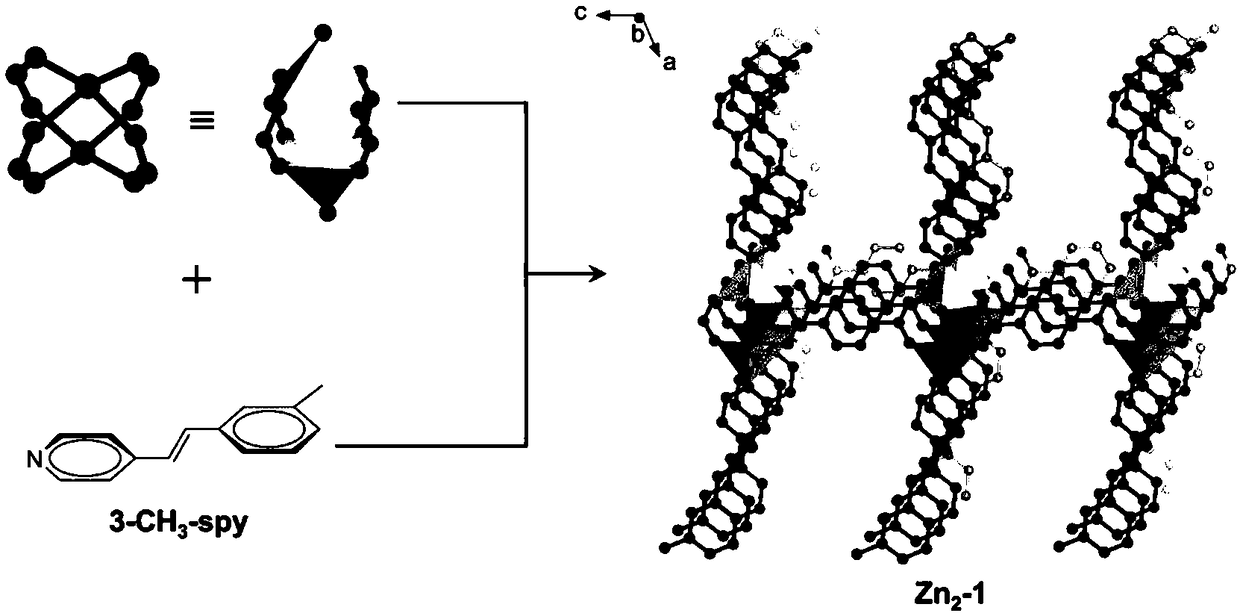
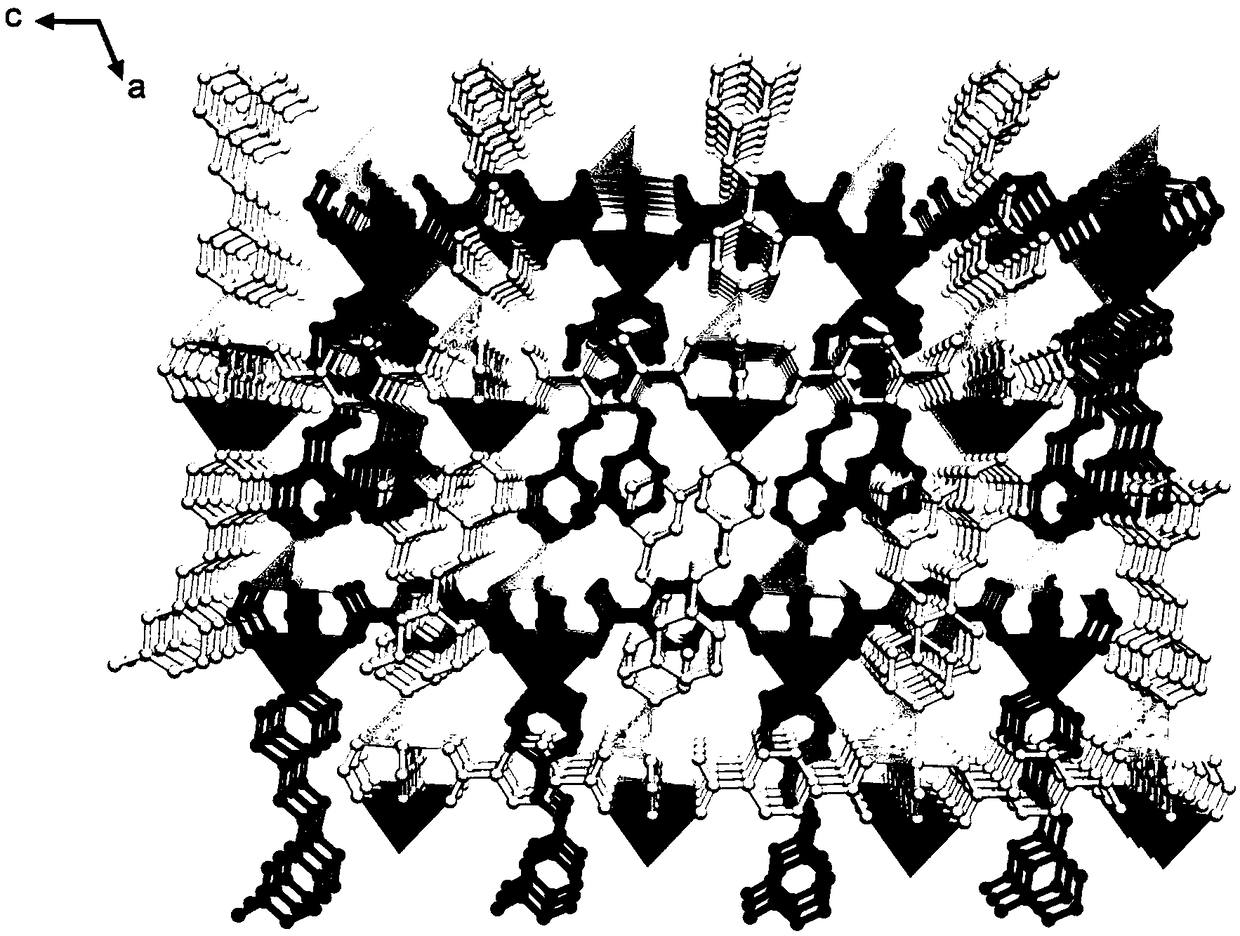
[0052] Example 1: metal organic framework compound [Zn 2 (bdc) 2 (3-CH 3 -spy)] H 2 Preparation of O
[0053] Charge a mixture of zinc sulfate heptahydrate (143 mg, 0.5 mmol), 3-methylstyryl-4-pyridine (97 mg, 0.5 mmol) and 1,4-terephthalic acid (83 mg, 0.5 mmol) into 25 mL Add 10 mL of a mixed solvent of N,N'-dimethylformamide and deionized water with a volume ratio of 2:3 into a thick-walled pressure-resistant bottle. Tighten the bottle cap and ultrasonically disperse for 10 minutes. Then it was placed in an oven whose temperature was programmed to rise to 145° C. and heated for 8 hours. Cool naturally to room temperature to obtain colorless rod-shaped crystals [Zn 2 (bdc) 2 (3-CH 3 -spy)] H 2 O(Zn 2 -1). The collection was washed with ethanol, and then dried in an oven at 60°C. Yield: 138.2 mg (63%, calculated based on 3-methylstyryl-4-pyridine).
[0054] Elemental analysis (%): C 44 h 36 N 2 o 9 Zn 2 ; Theoretical: C 60.92, H 4.18, N 3.23; Found: C 61.3, H...
Embodiment 2
[0060] Example two: metal organic framework compound [Zn 2 (bdc) 2 (rctt-tcdp)](Zn 2 - Preparation of 2a)
[0061] At room temperature, take a small amount of Zn 2 -1 crystal on a clean glass slide, with the distance between the light source and the crystal at 2cm, irradiated with a 420nm wavelength LED lamp for 30 minutes to obtain a 100% converted [2+2] cycloaddition product [Zn 2 (bdc) 2 (rctt-tcdp)](Zn 2 -2a).
[0062] Elemental analysis (%): C 44 h 34 N 2 o 8 Zn 2 , theoretical value: C 62.21, H 4.03, N 3.30; found value: C 61.73, H 4.16, N 3.28.
[0063] Infrared spectrum (potassium bromide tablet method): 3440(w), 3066(w), 2938(w), 2359(w), 1938(w), 1824(w), 1640(s), 1504(m) ,1393(s),1223(w),1032(m),919(w),881(m),821(s),745(s),688(m),546(s)cm -1 .
[0064] The product was subjected to X-ray single crystal diffraction test. Its crystallographic parameters are shown in Table 2, and the single crystal structure diagram is shown in the attached Figure 5 . ...
Embodiment 3
[0065] Example three: metal organic framework compound [Zn 2 (bdc) 2 (rctt-tcdp)](Zn 2 - Preparation of 2b)
[0066] At room temperature, take a small amount of Zn 2 -1 crystal on a clean glass slide, with the distance between the light source and the crystal at 2cm, irradiated with a 405nm wavelength LED lamp for 30 minutes to obtain a 100% converted [2+2] cycloaddition product [Zn 2 (bdc) 2 (rctt-tcdp)](Zn 2 -2b).
[0067] Elemental analysis (%): C 44 h 34 N 2 o 8 Zn 2 , theoretical value: C 62.21, H 4.03, N 3.30; found value: 63.09, H 4.11, N 3.39.
[0068] Infrared spectrum (potassium bromide tablet method): 3439(w), 3023(w), 2936(w), 1940(w), 1823(w), 1640(s), 1504(m), 1393(s) ,1223(w),1032(m),952(w),882(m),822(s),744(s),706(m),545(s)cm-1 .
[0069] The product was subjected to X-ray single crystal diffraction test, and its crystallographic parameters are shown in Table 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com