Antibody IgG against Staphylococcus aureus serine-rich repeat protein SraP and application
A staphylococcus and serine-rich technology, applied in the direction of anti-bacterial immunoglobulin, immunoglobulin, application, etc., can solve the problems of unsatisfactory effects and achieve high protective, high affinity, and high specific effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
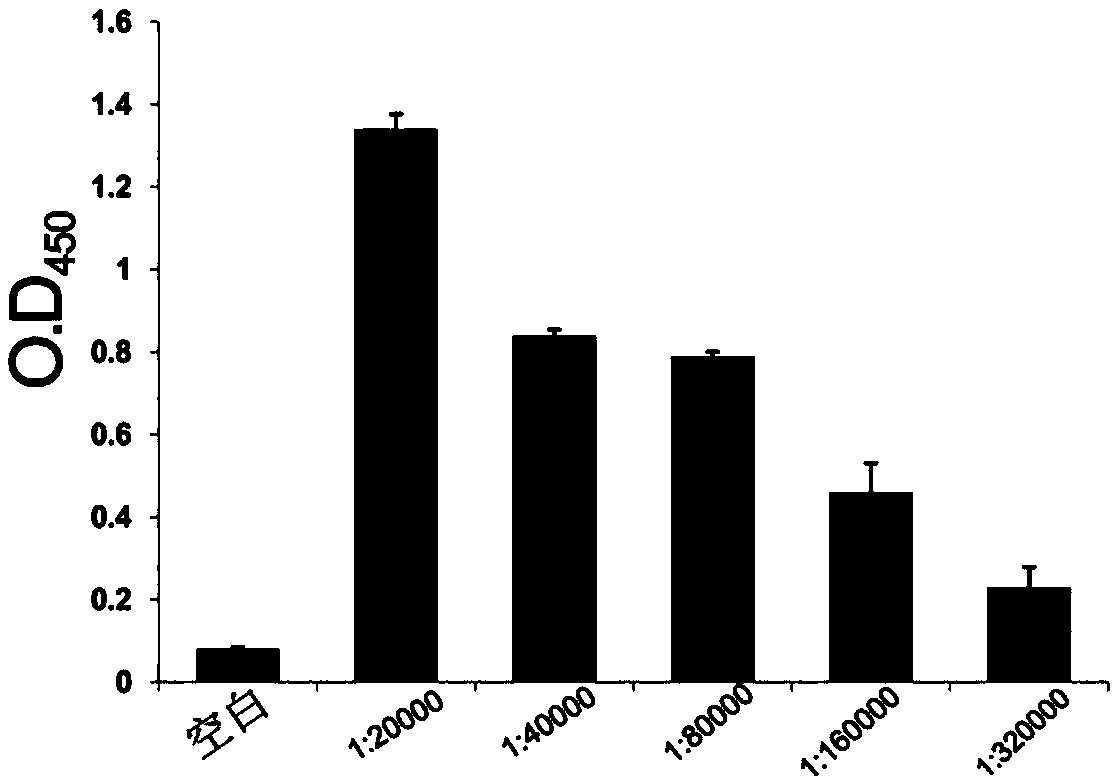
[0020] Example 1 Preparation and screening detection of mouse monoclonal antibody IgG
[0021] 1) Using hybridoma technology to prepare anti-SraP monoclonal antibody, the specific steps are as follows:
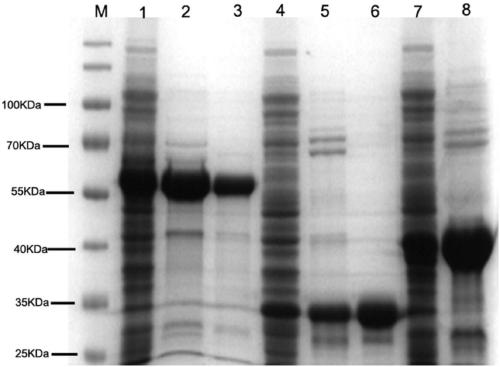
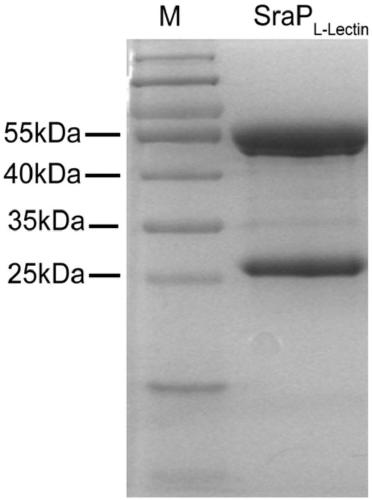
[0022] 1. Preparation of antigen (SraP L-Lectin Recombinant protein)
[0023] Build SraP L-Lectin Expression plasmid: extract the genome of Staphylococcus aureus USA300, follow the method of bacterial DNA extraction kit, and amplify SraP by PCR with the following primers L-Lectin Modular gene fragments,
[0024] F(5'-3'): CCGGATCCTTTGCGTCAGCAGCGACG;
[0025] R(5'-3'): CACAAGCTTTATATTCGAATGTTCCAAATTGTAC;
[0026] add at both ends BamHI and HindⅢ restriction endonuclease sites. Cloning the gene fragment into the pET28a expression vector to construct pET28a-SraP L-Lectin recombinant plasmid. The recombinant plasmid was successfully constructed by double enzyme digestion and DNA sequencing.
[0027]The above plasmid was transformed into Escherichia coli BL-21 competen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com