A method for preparing LPOR protein crystals
A protein and crystal technology, applied in the field of protein engineering, can solve the problems of unstable light-sensitive protein, loss and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] LPOR protein expression.
[0046]The plasmid pEBSRC-TEV-LPOR was transformed into C43 host cells, the plasmid pEBSRC-TEV-LPOR had Amp resistance, and the host C43 had no resistance. The medium used in the experiment was LB, and the plates were inverted and cultured overnight at 37°C. Pick the single clone on the transformation plate and transfer it to a 100mL Erlenmeyer flask containing 100mg / L Amp, culture overnight at 37°C and 220r / min, and transfer it to a 1L Erlenmeyer flask containing antibiotics on the second day with 1% inoculum , 37°C, 220r / min to continue culturing. After culturing for 4 h, IPTG was added with a final concentration of 0.4 mM to induce overnight. After the end of the induction, centrifuge at 6000r / min for 20min to collect the bacteria, discard the supernatant, and suspend the bacteria with buffer I.
Embodiment 2
[0048] Purification of LPOR protein
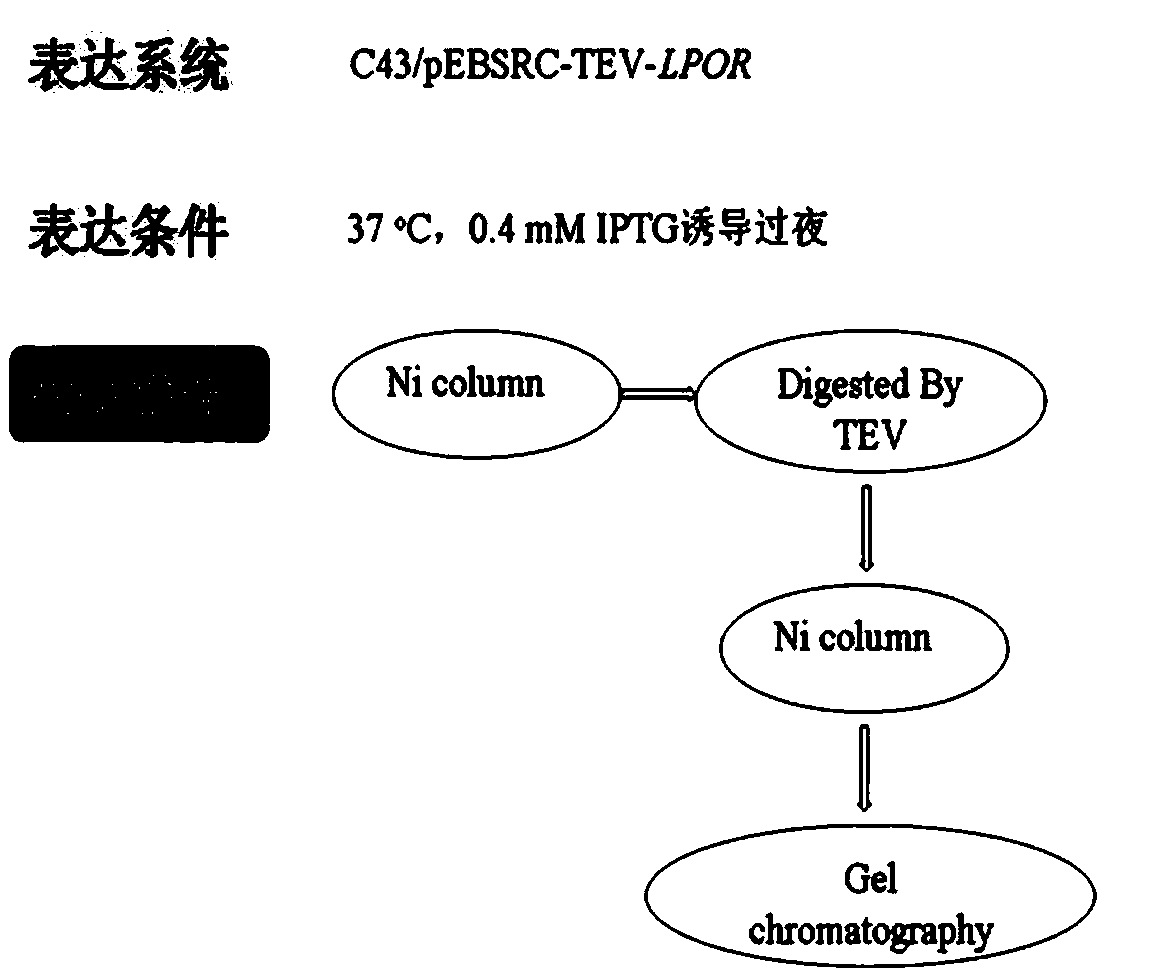
[0049] Such as figure 1 As shown, the specific steps for the purification of LPOR protein are as follows: the bacterial cell suspension is first crushed by a low-temperature and high-pressure cell crusher, and the crushing conditions are as follows: temperature 4°C, pressure 1000 MPa, crushing three times. The crushed solution was centrifuged at 16000r / min for 60 minutes to remove the cell debris in the precipitate, and the supernatant was the crude enzyme solution containing the target protein.
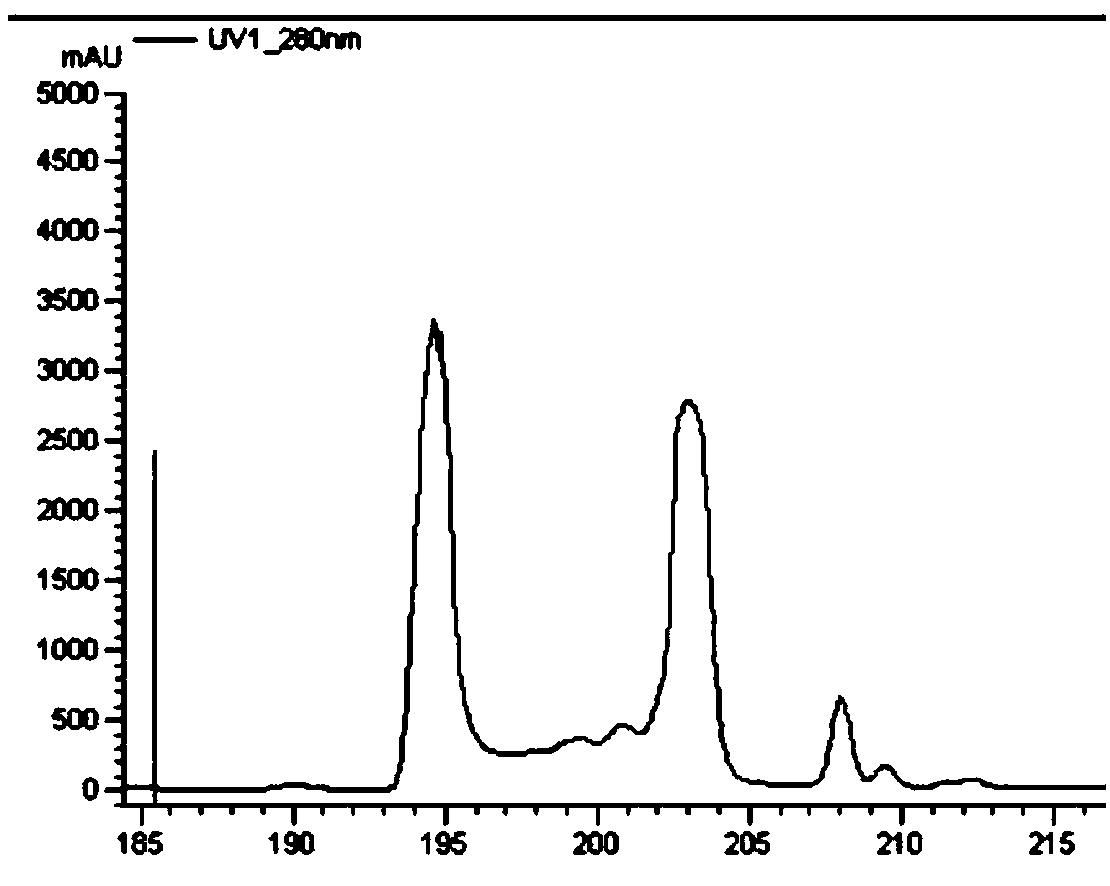
[0050] Pass the crude enzyme solution through the Ni affinity chromatography column, adsorb the target protein on the Ni column, then use buffer I to equilibrate the purification column, and then use buffer II, buffer III and buffer IV to wash the purification column respectively, and each eluted protein The peaks were collected separately for verification by SDS-PAGE, in which LPOR was eluted by buffer III, and the buffer III eluted protein was...
Embodiment 3
[0058] Crystallization of LPOR proteins
[0059] Use Amicon Ultra-30k ultrafiltration membrane to concentrate protein, and use Nanodrop to measure the concentration to be 30mg / mL. After taking 1 μL of concentrated protein for SDS-PAGE to verify the purity and concentration, use conventional crystallization buffer (2-methyl-2, 4-pentanediol) began to point crystals, and the manipulator was used to screen, and a large number of crystal points were carried out for the crystallization conditions of long crystals;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com