Synthesis and use of 9-O-aryl substituted berberine derivatives
A technology of berberine and its derivatives, which is applied in the synthesis method and its antibacterial application field, can solve the problems of rarely used modification, etc., and achieve the effect of wide range of drug effects, wide substrate applicability, and excellent physiological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053]
[0054] (1) Synthesis of Berberine
[0055] Take 5.4 mmol of berberine, place it in a round-bottomed flask, heat it under vacuum at 20-30 mmHg, 190°C for 1 h, cool to room temperature after the reaction, and obtain 4.8 mmol of berberine 2 with a yield of 90%, without purification Proceed directly to the next reaction.
[0056] (2) Synthesis of Tetrahydroberberine
[0057] Take 6.0 mmol of berberine, put it in a round bottom flask, add HCl / EtOH (1:10) solution and stir at room temperature for 1 h, after the reaction is completed, filter and dry to obtain protonated tetrahydroberberine, Yield 95%. Take 2.0 mmol of protonated tetrahydroberbererythrine, put it in a round bottom flask, add methanol to dissolve it, and then add 10.0 mmol of NaBH in batches under a water bath 4 , the addition was completed, stirred overnight at 50° C., the reaction was completed, filtered, washed with methanol, dissolved in ethyl acetate, and the ethyl acetate was spun off under reduced...
Embodiment 2
[0066]
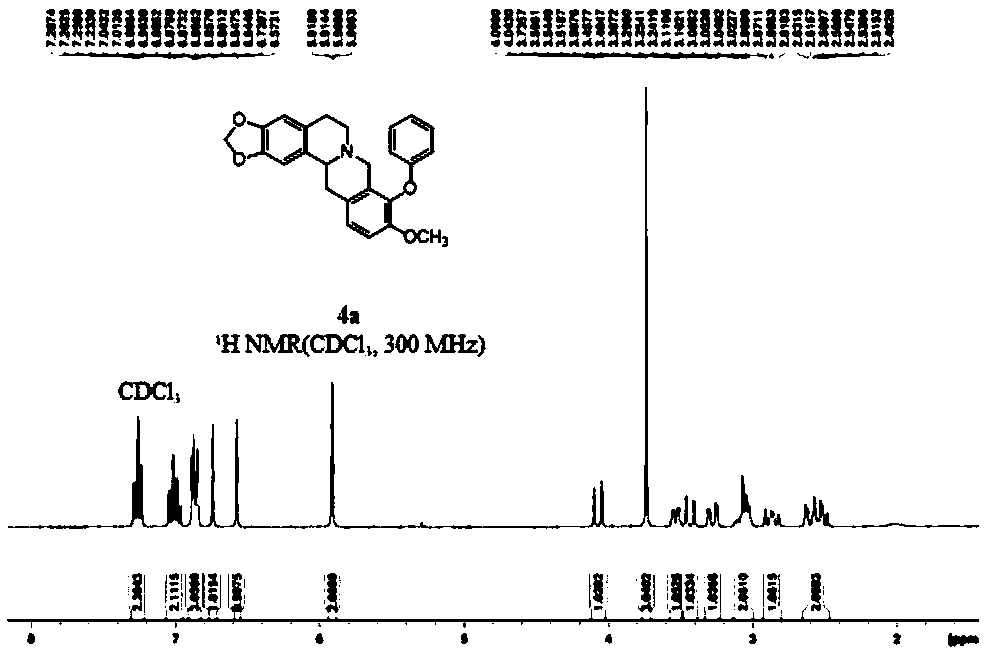
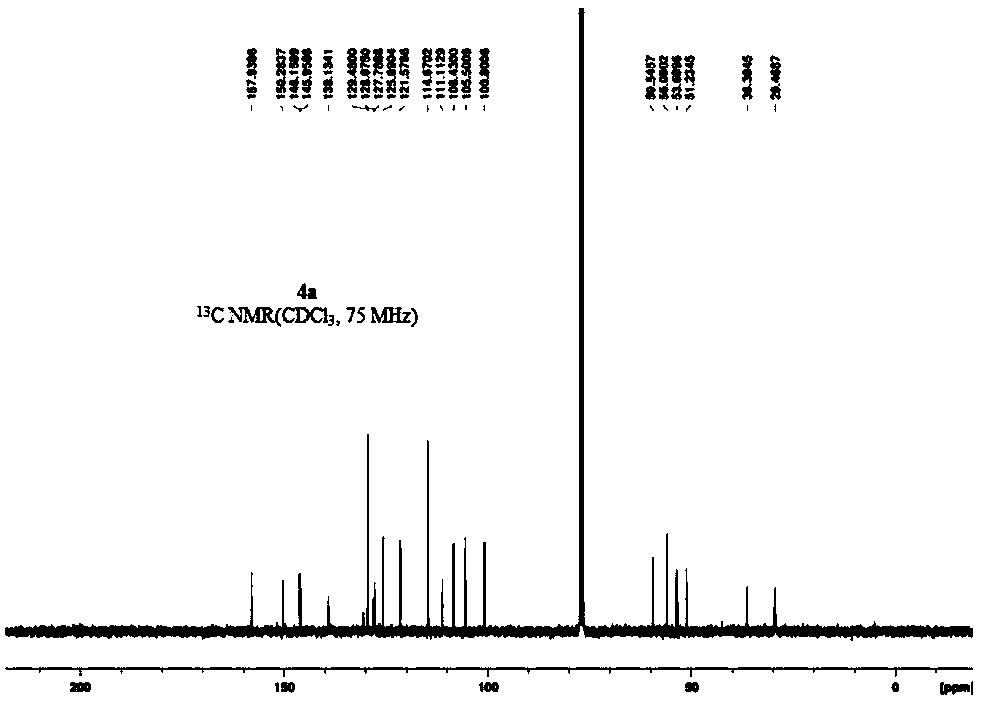
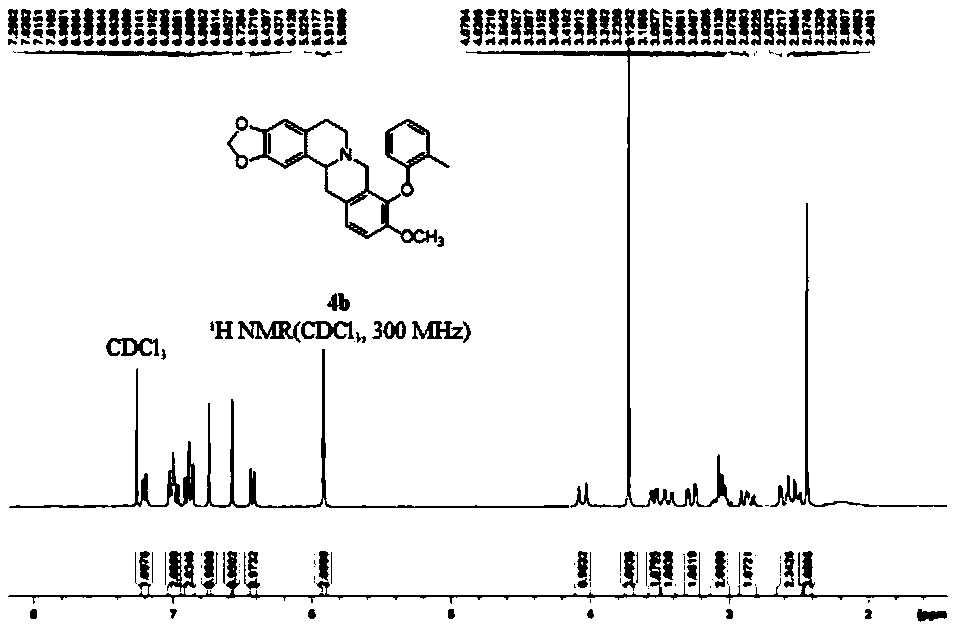
[0067] 4b: Pale yellow solid, 69% yield, mp 153-155°C. 1 H NMR (300MHz, CDCl 3 ):δ7.20(dd,1H,J=0.8Hz,J=7.3Hz,Ar-H),7.03-6.96(m,2H,Ar-H),6.91-6.85(m,2H,Ar-H) ,6.74(s,1H,H-1),6.57(s,1H,H-4),6.44-6.41(m,1H,Ar-H),5.92(q,2H,J=1.4Hz,-OCH 2 O-), 4.05(d, 1H, J=15.8Hz, H-8), 3.72(s, 3H, H-10), 3.56-3.52(m, 1H, H-14), 3.43(d, 1H, J=15.8Hz, H-8), 3.30-3.24(m, 1H, H-13), 3.12-3.03(m, 2H, H-6&H-5), 2.91-2.82(m, 1H, H-5) ,2.64-2.48(m,2H,H-6&H-13); 13 C{ 1 H}NMR (75MHz, CDCl 3 ): δ156.0, 150.3, 146.2, 146.0, 139.8, 130.9, 130.7, 129.3, 128.1, 127.8, 126.7, 126.1, 125.4, 121.3, 112.3, 111.3, 108.4, 105.5, 100.8 (-OCH 2 O-), 59.5 (C-14), 56.2 (10-OCH 3 ), 53.6(C-8), 51.2(C-6), 36.3(C-13), 29.4(C-5), 16.3(-CH 3 ); HRMS(ESI): m / z for C 26 h 26 NO 4 + [M+1] + calcd 416.1856, found 416.1861.
[0068] 5b: Yellow solid, 84% yield, mp 252-254°C. 1 H NMR (300MHz, DMSO-d 6 ):δ9.78(s,1H,H-8),9.07(s,1H,H-13),8.30(d,1H,J=9.2Hz,H-12),8.20(d,1H,J= 9.2Hz, H-11),7.82(s,1H,H-1),7.35...
Embodiment 3
[0070]
[0071] 4c: Pale yellow solid, 90% yield, mp 158-160°C. 1 H NMR (300MHz, CDCl 3 ): δ7.12(t, 1H, J=7.8Hz, Ar-H), 7.02(d, 1H, J=8.4Hz, H-11), 6.87(d, 1H, J=8.4Hz, H-12 ),6.80(d,1H,J=7.5Hz,Ar-H),6.73(s,1H,H-1),6.70-6.69(m,1H,Ar-H),6.62(dd,1H,J= 2.4Hz, J=8.2Hz, Ar-H), 6.57(s, 1H, H-4), 5.92(q, 2H, J=1.4Hz, -OCH 2 O-), 4.06(d, 1H, J=15.9Hz, H-8), 3.74(s, 3H, H-10), 3.56-3.51(m, 1H, H-14), 3.43(d, 1H, J=15.9Hz, H-8), 3.30-3.24(m, 1H, H-13), 3.11-2.99(m, 2H, H-6&H-5), 2.91-2.81(m, 1H, H-5) ,2.62-2.49(m,2H,H-6&H-13); 13 C{ 1 H}NMR (75MHz, CDCl 3 ):δ157.9,150.3,146.2,145.9,139.6,139.2,130.7,129.6,129.2,128.1,127.8,125.6,122.4,115.4,111.5,111.2,108.4,105.5,100.8(-OCH 2 O-), 59.5 (C-14), 56.1 (10-OCH 3 ), 53.7(C-8), 51.2(C-6), 36.4(C-13), 29.5(C-5), 21.5(-CH 3 ); HRMS(ESI): m / z for C 26 h 26 NO 4 + [M+1] + calcd 416.1856, found 416.1862.
[0072] 5c: Yellow solid, 93% yield, mp 239-241°C. 1 H NMR (300MHz, DMSO-d 6 ):δ9.77(s,1H,H-8),9.07(s,1H,H-13),8.31(d,1H,J=9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com