A spiropyran-bipyridine derivative and its naked-eye detection of copper(ii)
A derivative, spiropyran technology, applied in the preparation of biological probe materials and its application field, can solve the problems of poor water solubility, light sensitivity of spiropyran, complicated operation, etc., achieve rapid response, realize naked eye detection, dissolve good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
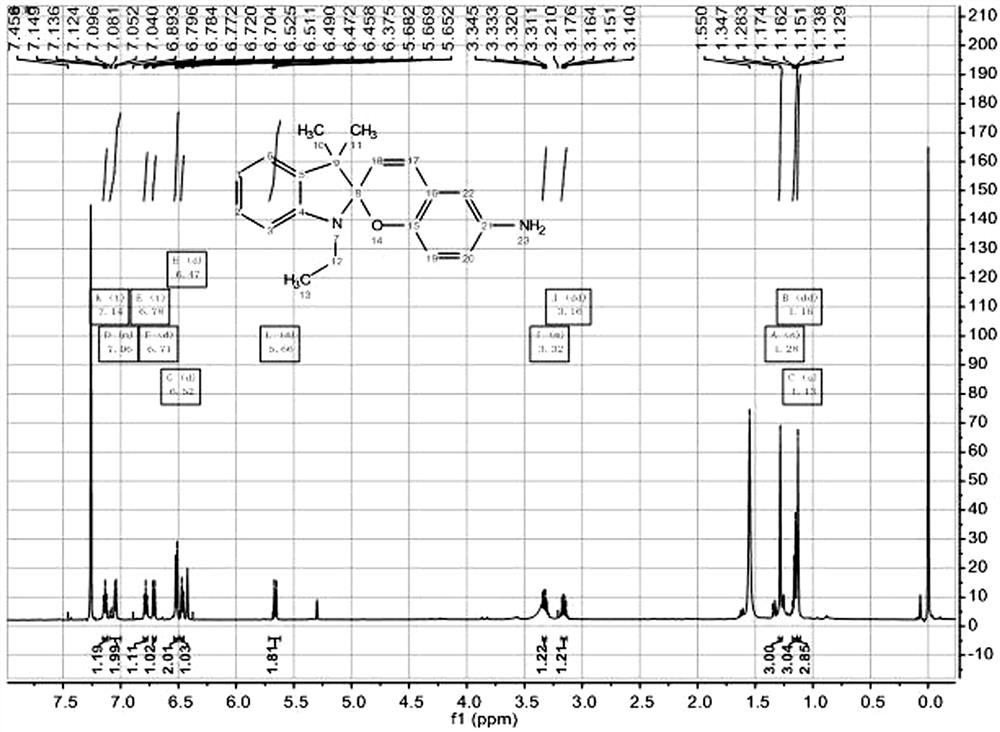
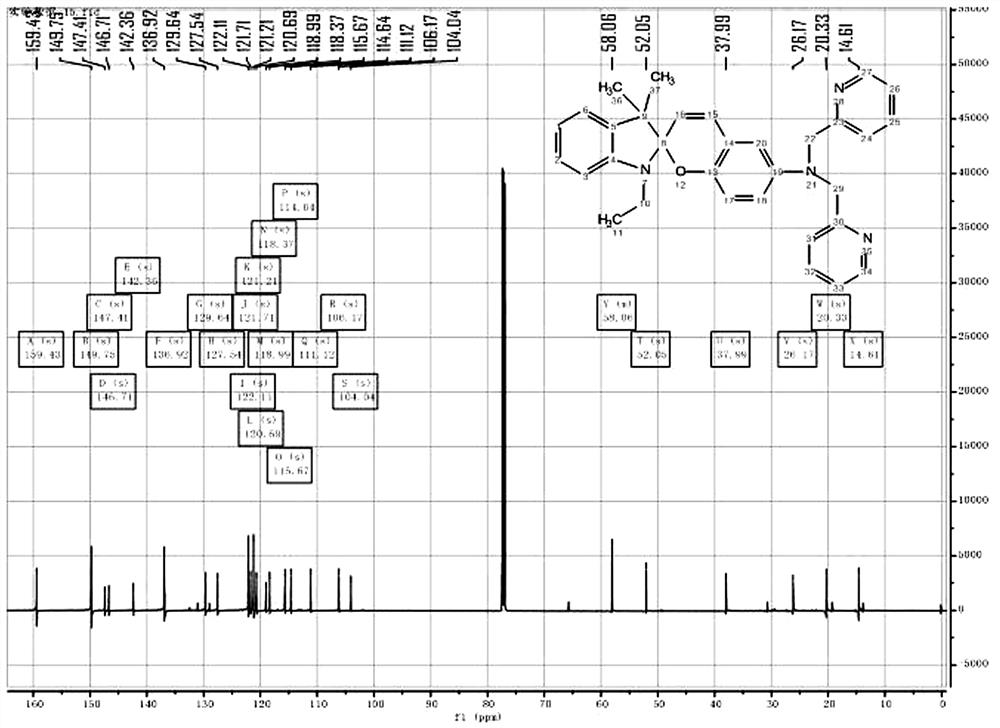
[0020] Embodiment 1: a kind of spiropyran-bipyridine derivative, the structural formula of the probe compound is:
[0021] , abbreviated as SP-DP.
[0022] The method for preparing the spiropyran-bispyridine derivatives, heat reflux of semicyanine and ethyl bromide in acetonitrile solvent to generate semicyanine derivatives, which generate yellow oily liquid under the action of alkali , the oily liquid and 5-nitrosalicylaldehyde are heated under reflux in ethanol to form a ring-forming reaction to generate spiropyranyl nitro derivatives, and the spiropyranyl nitro derivatives are reduced to spiropyranyl under the action of stannous chloride and acetic acid Nylamino derivatives, spiropyranyl amino derivatives and twice the amount of chloromethylpyridine under the action of potassium carbonate and potassium iodide, the two hydrogens on the amino group are replaced by pyridine rings to generate spiropyridine with two pyridine rings Fyran-bipyridine derivative SP-DP.
[0023] ...
Embodiment 2
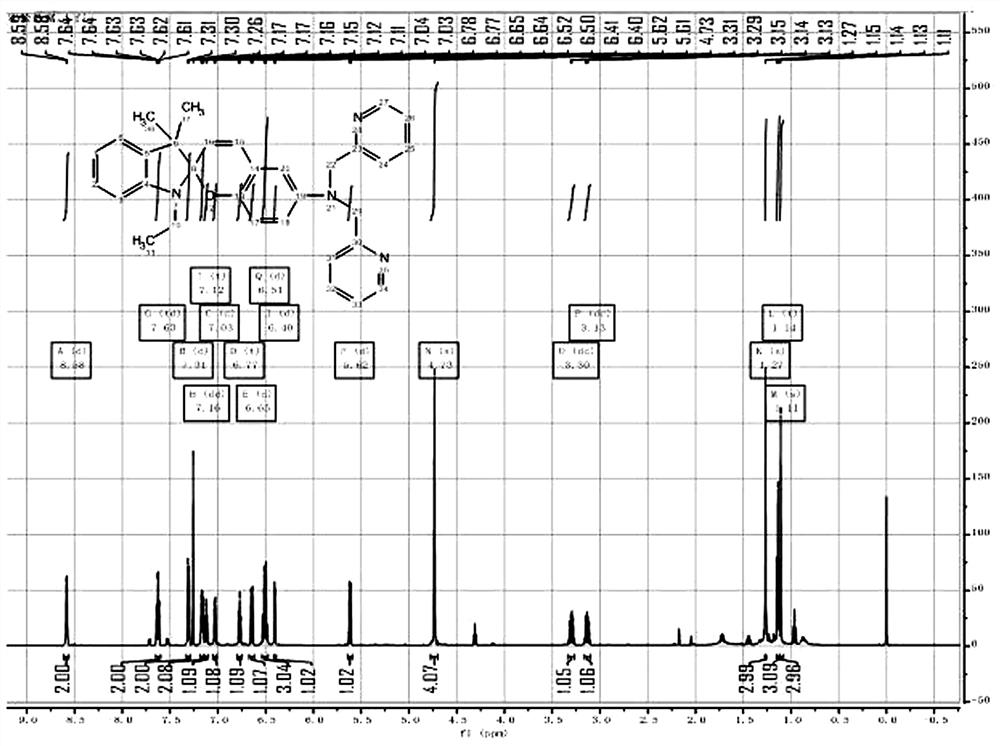
[0028] Embodiment 2: spiropyran-bipyridine probe detects Cu 2+ :
[0029] Preparation of spiropyran-bipyridine probe mother solution: DMSO was used as a solvent to prepare a spiropyran-bipyridine probe molecule mother solution with a final concentration of 2 mM.
[0030] Take 20µL of the mother solution of the probe molecule SP-DP and 1980µL of PB (pH=7.4, 50 mM) in the cuvette, so that the final concentration is 20 µM, and measure the UV-Vis absorption spectrum. It can be seen that there is a The absorption peak of the spiropyran ring. After adding 1 equivalent of Cu 2+ , a new peak appears at 517 nm in the UV absorption, which indicates that the Cu 2+ Combined with spiropyran probe molecules in a certain way to generate new substances.
[0031] In the detection with Cu 2+ Under exactly the same conditions, for other common metal ions (Fe 3+ , Zn 2+ , Mn 2+ 、Co 2+ , Hg 2+ 、Cd 2+ , Sn 2+ 、Ni 2+ 、Cr 2+ 、Ag + , Fe 2+ ) were detected. Take 20 µL of the mother sol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com