Chair type copper-iodine cluster and nitrophenol selective sensing application thereof
A technology of o-nitrophenol and high selectivity, which is applied in the direction of copper organic compounds, 1/11 group organic compounds without C-metal bonds, instruments, etc. It can solve the problems of low selectivity and complex sensor synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
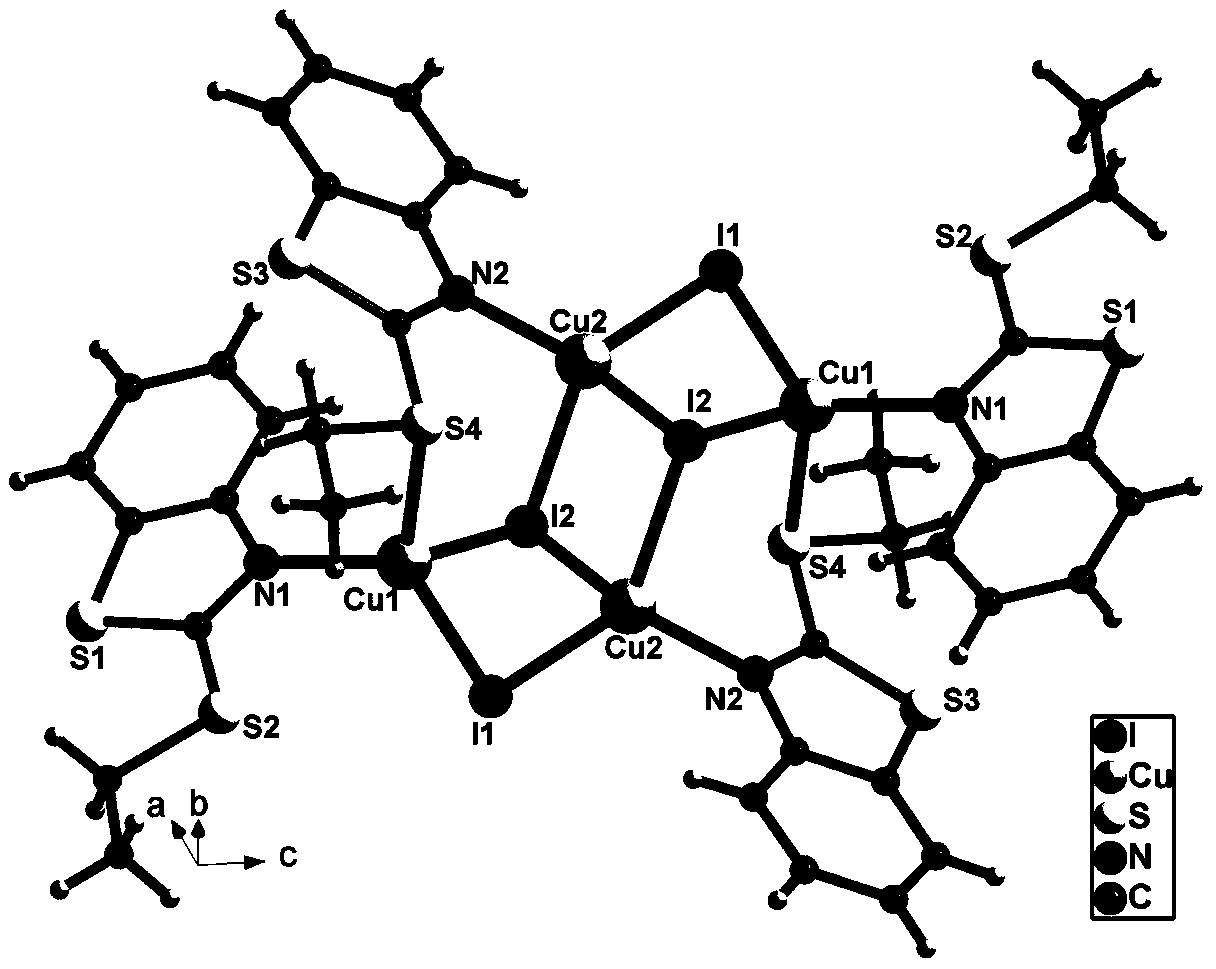
[0010] We chose cuprous iodide and 2-mercaptobenzothiazole as the reaction raw materials, ethanol, acetonitrile and hydroiodic acid as the reaction raw materials or solvents, and obtained the compound Cu under solvothermal conditions. 4 I 4 (Etmbt) 4 single crystal. The organic component Etmbt is produced by the in-situ ethylation of the raw material 2-mercaptobenzothiazole under acidic conditions.
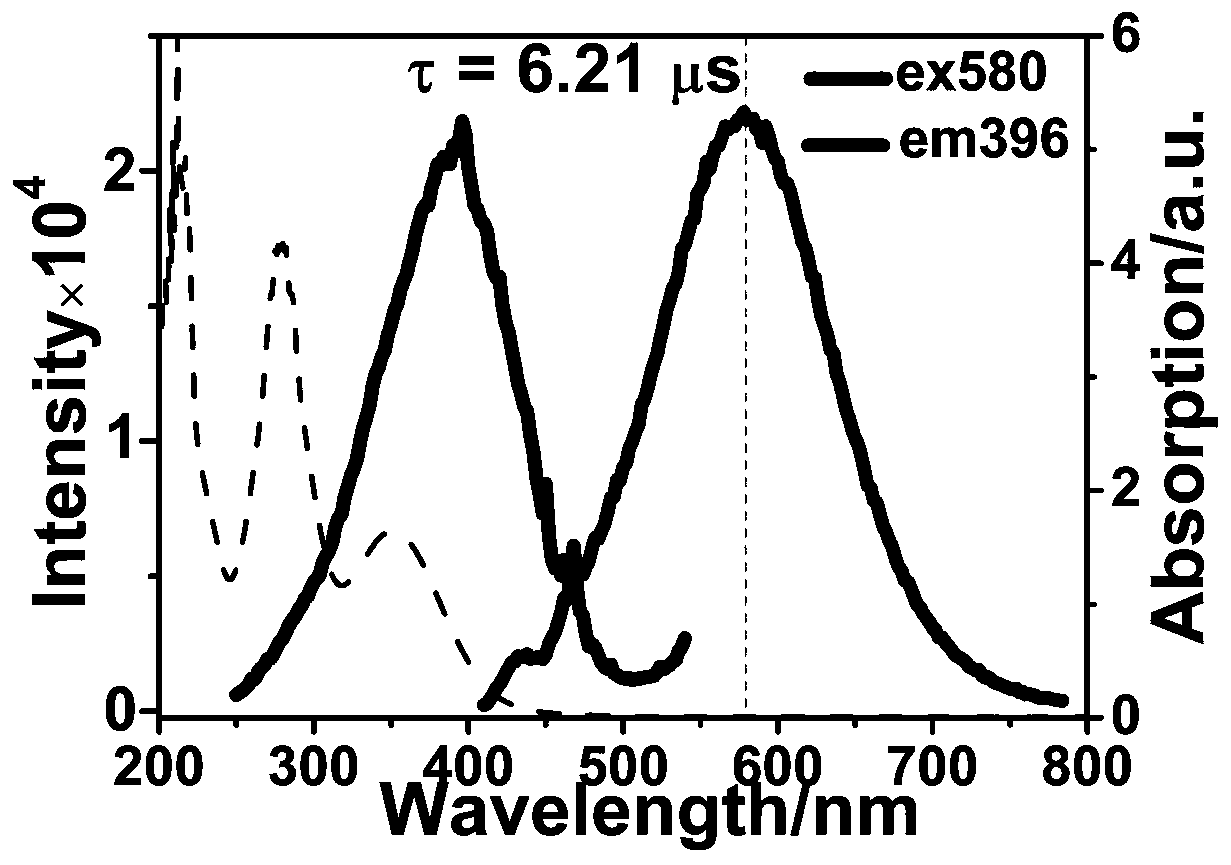
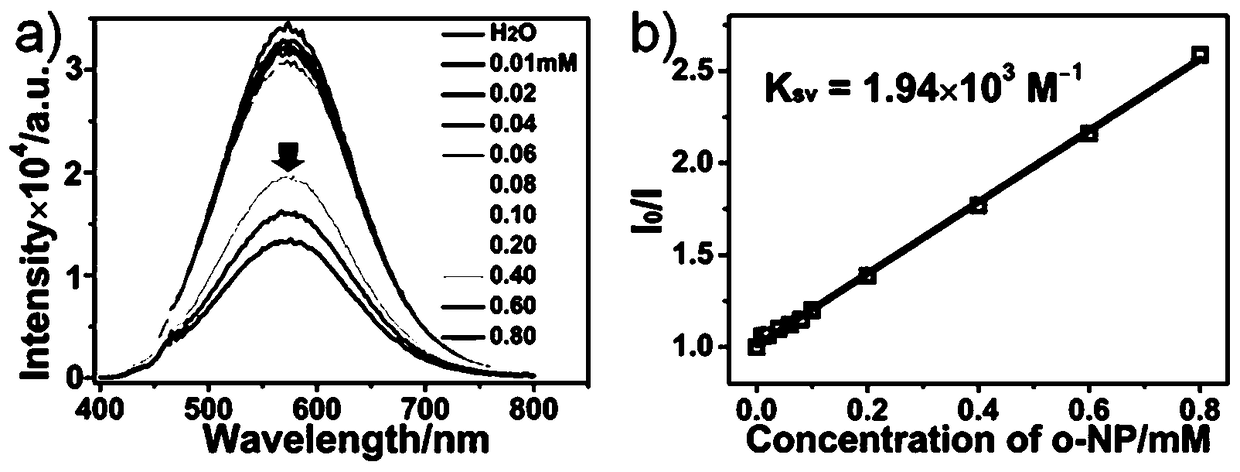
[0011] The beneficial effect of the present invention is that the synthetic raw material of the involved tetra-nuclear copper-iodine cluster material is non-toxic, easy to obtain, simple in synthesis thinking, easy to operate and low in cost. The tetranuclear copper-iodine cluster material involved in this invention has excellent photoluminescence properties, and can detect o-nitrophenol with high selectivity in water systems, and is not affected by its isomers m-nitrophenol and p-nitrophenol. , and the interference of other common organic substances, with high sensitivity and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com