Joint test strip and preparation method thereof
A technology of test strips and joint inspection tests, applied in the field of joint inspection test strips and its preparation, can solve the problems of low test results, easy hook effect, false negatives, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
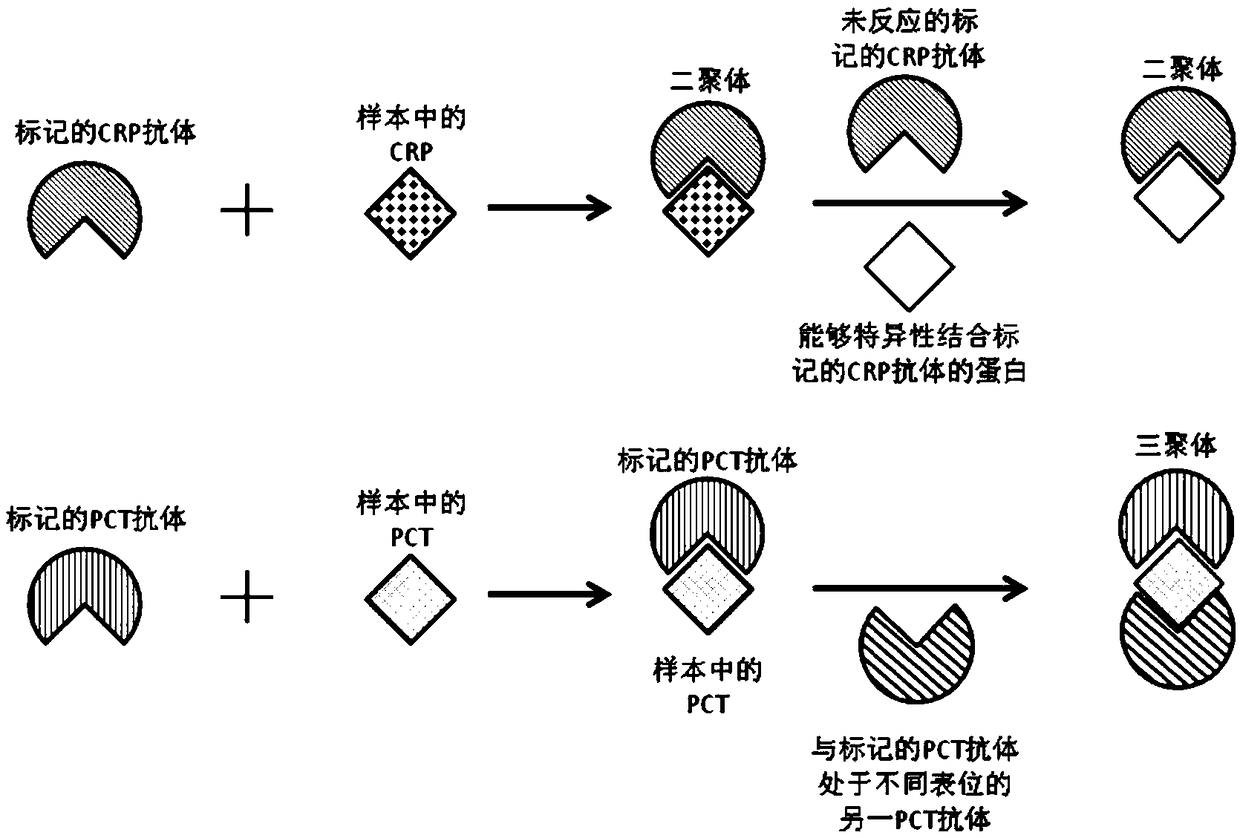
[0066] This embodiment provides a C-reactive protein and procalcitonin combined detection test strip and its preparation method.
[0067] 1. Preparation of joint test strips
[0068] (1) Sample pad preparation
[0069] The diluent is a PBS solution containing 0.3% BSA, 1.2% Tween 20, 6% sucrose, 3% sorbitol, 0.08% sodium azide, 0.03M, pH7.4, where "%" refers to the mass percentage.
[0070] In this embodiment, fluorescent microspheres with a diameter of 200-500 nm are mainly used as the marker substance, and the marker substance will generate emitted light with a Stokes shift of about 50-100 nm after being excited by light.
[0071] Activate the labeling substance by conventional methods (sonication for 30-60 seconds), covalently couple it with an antibody with a protein concentration of 0.2-1.0 mg / mL, react at room temperature for 2 hours, centrifuge and wash twice, each time 8000-15000×g , Centrifuge for 10-15 minutes, and finally dissolve the precipitate with PBS contain...
Embodiment 2
[0115] This embodiment is a modification example of embodiment 1, providing a joint inspection test strip and its preparation method. The changes of this example relative to embodiment 1 include the preparation steps of the joint inspection test strip:
[0116] (1) Sample pad preparation
[0117] The diluent is 0.03M, pH7.4 PBS containing 0.1% BSA, 0.5% Tween 20, 0.5% Tween 80, 2% sucrose, 1% sorbitol, 0.05% sodium azide, where "% " refers to mass percentage.
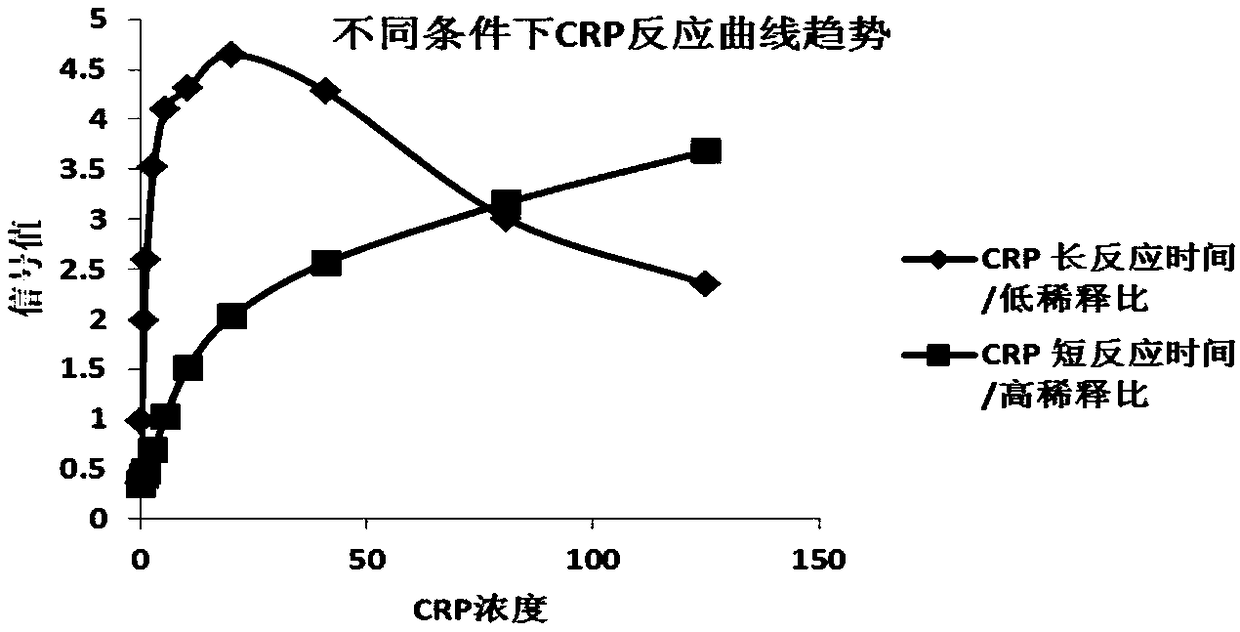
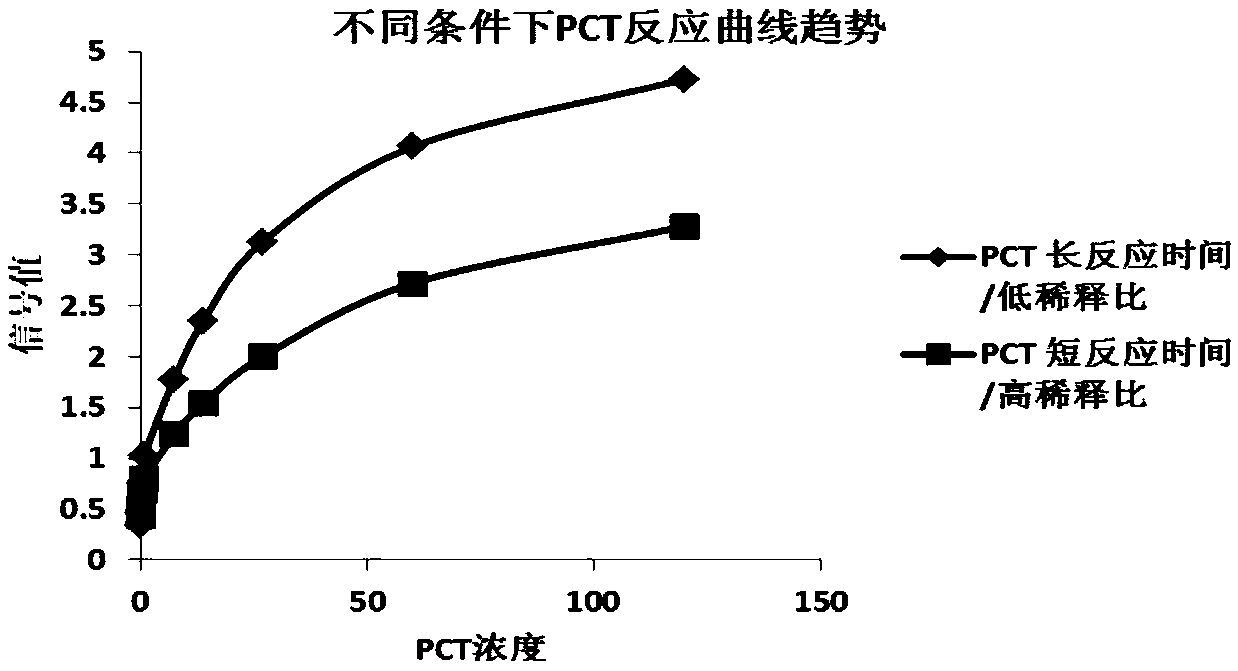
[0118] The labeled PCT antibody, labeled CRP antibody, and labeled quality control protein (labeled anti-chicken IgY) were respectively adjusted according to the proportions of 5%, 5%, and 0.5% (adjusted according to the actual experimental signal intensity and the trend of the reaction curve), using the above-mentioned After the diluent is diluted, it is evenly sprayed on the sample pad with a gold spraying device, and the spraying volume is 2 μL / cm respectively. After the sprayed sample mat is dried under the condit...
Embodiment 3
[0126] This embodiment is a modification example of embodiment 1, providing a joint inspection test strip and its preparation method. The changes of this example relative to embodiment 1 include the preparation steps of the joint inspection test strip:
[0127] (1) Sample pad preparation
[0128]Diluents include: 0.5% BSA, 2% Tween 20, 1% Tween 80, 10% sucrose, 5% sorbitol, 0.1% sodium azide, constant volume with 0.03M, pH7.4 PBS, where "%" means mass percentage.
[0129] The labeled antibody of PCT, the labeled antibody of CRP, and the labeled quality control protein (labeled anti-mouse IgG) were respectively adjusted according to the ratio of 10%, 10%, and 2% (adjusted according to the actual experimental signal intensity and the trend of the reaction curve). After the diluent is diluted, it is evenly sprayed on the sample pad using a gold spraying device. After the sprayed sample pad is dried under the condition of relative humidity <20% and 37°C, it is sealed and dried a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com