Medicinal preparation for treating hepatobiliary diseases and preparation method thereof
A technology for pharmaceutical preparations and hepatobiliary diseases is applied in the field of pharmaceutical preparations for the treatment of hepatobiliary diseases and their preparation, and can solve the problems affecting the quality and curative effect of ursodeoxycholic acid tablets, production processes, differences in excipients and components, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
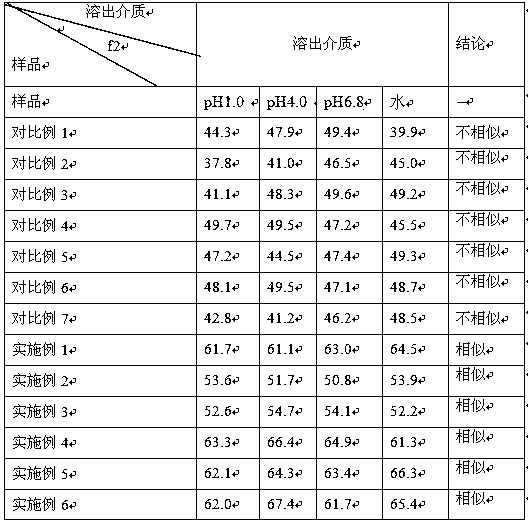
Examples
Embodiment 1
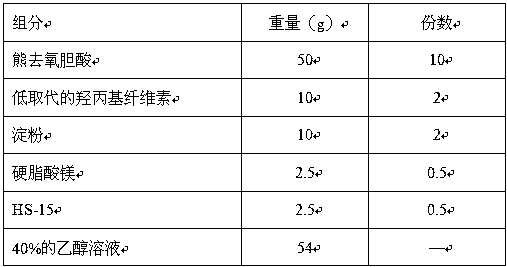
[0032] Example 1: Ursodeoxycholic Acid Tablets
[0033] Prescription composition (1000 tablets):
[0034]
[0035] Preparation:
[0036] ① Micronization treatment of raw materials: Micronize the raw materials of ursodeoxycholic acid to obtain more than 90% of the micropowder with a particle size of no more than 40-50 microns, and set aside;
[0037] ② Pretreatment of excipients: hydroxypropyl cellulose, starch, and magnesium stearate are respectively passed through a 120-mesh sieve, and set aside; dissolve HS-15 with 35% ethanol to prepare a binder solution, set aside;
[0038] ③ Mixing raw and auxiliary materials: mix ursodeoxycholic acid, starch, and hydroxypropyl cellulose evenly;
[0039] ④ Soft material granulation: add the powder in step ③ to the binder solution, stir to prepare soft material; take the prepared soft material and pass through a 14-mesh sieve to granulate.
[0040] ⑤ Drying and granulation: Take the prepared granules and dry them, then granulate the ...
Embodiment 2
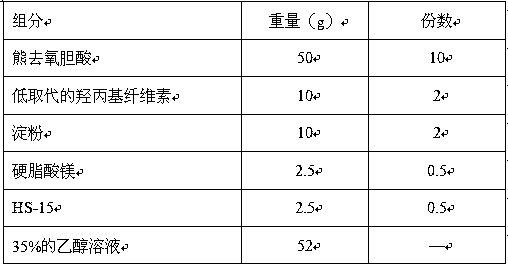
[0042] Example 2: Ursodeoxycholic Acid Tablets
[0043] Prescription composition (1000 tablets):
[0044]
[0045] Preparation:
[0046] ① Micronization treatment of raw material drug: micronized ursodeoxycholic acid raw material drug to obtain more than 90% of the micropowder with a particle size of no more than 40-50 microns, and set aside;
[0047] ②Excipient material pretreatment: hydroxypropyl cellulose, starch, and magnesium stearate are respectively passed through a 100-mesh sieve, and set aside; dissolve HS-15 with 40% ethanol to prepare a binder solution, set aside;
[0048] ③ Mixing raw and auxiliary materials: mix ursodeoxycholic acid, starch, and hydroxypropyl cellulose evenly;
[0049] ④ Soft material granulation: add the powder in step ③ to the binder solution, stir to prepare soft material; take the prepared soft material and pass through a 14-mesh sieve to granulate.
[0050] ⑤ Drying and granulation: Take the prepared granules and dry them, then granulat...
Embodiment 3
[0052] Example 3: Ursodeoxycholic Acid Tablets
[0053] Prescription composition (1000 tablets):
[0054] components
Weight (g)
number of copies
ursodeoxycholic acid
50
10
low substituted hydroxypropyl cellulose
12.5
2.5
starch
9
1.8
Magnesium stearate
2.5
0.5
HS-15
1
0.2
38% ethanol solution
53
—
[0055] Preparation:
[0056] ① Micronization treatment of raw material drug: micronized ursodeoxycholic acid raw material drug to obtain more than 90% of the micropowder with a particle size of no more than 40-50 microns, and set aside;
[0057]②Excipient material pretreatment: hydroxypropyl cellulose, starch, and magnesium stearate are respectively passed through a 100-mesh sieve, and set aside; dissolve HS-15 with 38% ethanol to prepare a binder solution, set aside;
[0058] ③ Mixing raw and auxiliary materials: mix ursodeoxycholic acid, starch, and hydroxypropyl cellulose evenly...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com