A kind of pharmaceutical preparation for treating liver and gallbladder disease and preparation method thereof
A technology for pharmaceutical preparations and liver and gallbladder diseases, which is applied in the field of pharmaceutical preparations for the treatment of liver and gallbladder diseases and its preparation, and can solve problems affecting the quality and curative effect of ursodeoxycholic acid tablets, production processes, and differences in excipient ingredients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Ursodeoxycholic Acid Tablets
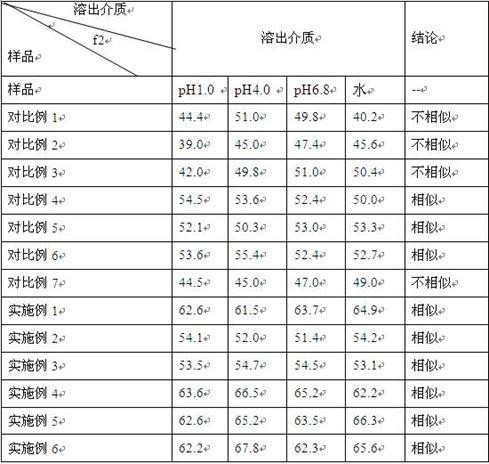
[0033] Prescription composition (1000 tablets):
[0034]
[0035] Preparation:
[0036] ① Micronization treatment of raw materials: Micronize the raw materials of ursodeoxycholic acid to obtain more than 90% of the micropowder with a particle size of no more than 40-50 microns, and set aside;
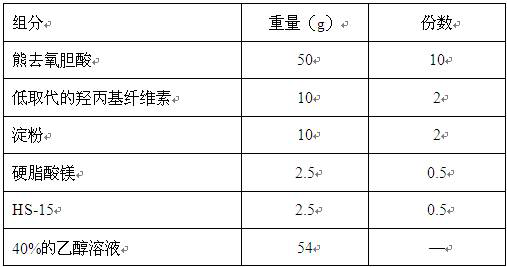
[0037] ② Pretreatment of excipients: hydroxypropyl cellulose, starch, and magnesium stearate are respectively passed through a 120-mesh sieve, and set aside; HS-15 is dissolved in 35% ethanol to prepare a binder solution, set aside;
[0038] ③ Mixing raw and auxiliary materials: mix ursodeoxycholic acid, starch, and hydroxypropyl cellulose evenly;
[0039] ④ Soft material granulation: add the powder in step ③ to the binder solution, stir to prepare soft material; take the prepared soft material and pass through a 14-mesh sieve to granulate.
[0040] ⑤ Drying and granulation: Take the prepared granules and dry them, then granulate th...
Embodiment 2
[0042] Example 2: Ursodeoxycholic Acid Tablets
[0043] Prescription composition (1000 tablets):
[0044]
[0045] Preparation:
[0046] ① Micronization treatment of raw material drug: micronized ursodeoxycholic acid raw material drug to obtain more than 90% of the micropowder with a particle size of no more than 40-50 microns, and set aside;
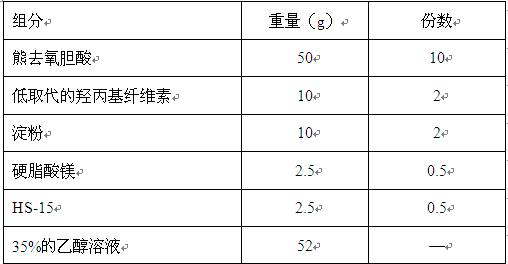
[0047] ②Excipient material pretreatment: hydroxypropyl cellulose, starch, and magnesium stearate are respectively passed through a 100-mesh sieve, and set aside; 40% ethanol is used to dissolve HS-15 to prepare a binder solution, set aside;
[0048] ③ Mixing raw and auxiliary materials: mix ursodeoxycholic acid, starch, and hydroxypropyl cellulose evenly;
[0049] ④ Soft material granulation: add the powder in step ③ to the binder solution, stir to prepare soft material; take the prepared soft material and pass through a 14-mesh sieve to granulate.
[0050] ⑤ Drying and granulation: Take the prepared granules and dry them, then gr...
Embodiment 3
[0052] Example 3: Ursodeoxycholic Acid Tablets
[0053] Prescription composition (1000 tablets):
[0054] components Weight (g) number of copies ursodeoxycholic acid 50 10 low substituted hydroxypropyl cellulose 12.5 2.5 starch 9 1.8 Magnesium stearate 2.5 0.5 HS-15 1 0.2 38% ethanol solution 53 —
[0055] Preparation:
[0056] ① Micronization treatment of raw material drug: micronized ursodeoxycholic acid raw material drug to obtain more than 90% of the micropowder with a particle size of no more than 40-50 microns, and set aside;
[0057]②Excipient material pretreatment: hydroxypropyl cellulose, starch, and magnesium stearate are respectively passed through a 100-mesh sieve, and set aside; dissolve HS-15 with 38% ethanol to prepare a binder solution, set aside;
[0058] ③ Mixing raw and auxiliary materials: mix ursodeoxycholic acid, starch, and hydroxypropyl cellulose evenly;
[0059] ④ Soft material granulation: ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com