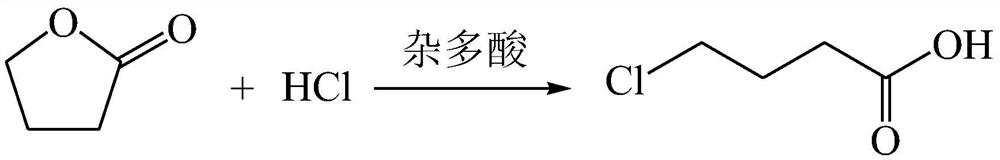
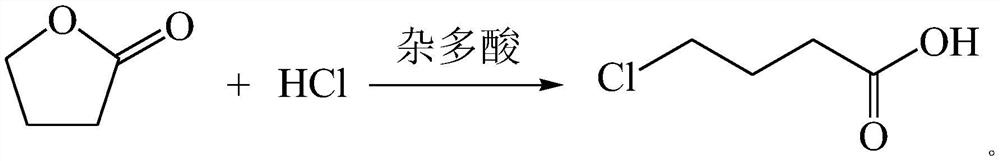
A method for synthesizing gamma-chlorobutyric acid
A technology of chlorobutyric acid and butyrolactone, applied in the preparation of carboxylate/lactone, organic chemistry, etc., can solve the problems of high operation requirements, potential safety hazards, etc., to avoid high-pressure reaction, avoid danger, and yield high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Put 86 g of γ-butyrolactone into the flask, and keep stirring at room temperature and normal pressure. Add 5g of silicotungstic acid into the flask, keep stirring at room temperature and normal pressure. 200g of 20% hydrochloric acid was slowly added dropwise, and the reaction was stirred for 2h. Sampling and GC analysis showed that the residual gamma-butyrolactone was less than 1%, and the gamma-chlorobutyric acid content reached more than 99%. Leave to stand for stratification, collect the product of the lower layer, and repeat the application of the upper layer of water.
Embodiment 2
[0018] Put 86 g of γ-butyrolactone into the flask, and keep stirring at room temperature and normal pressure. Add 4.3g of phosphotungstic acid into the flask, keep stirring at room temperature and normal pressure. 200g of 20% hydrochloric acid was added dropwise, and the reaction was stirred for 2h. Sampling and GC analysis showed that the residual gamma-butyrolactone was less than 1%, and the gamma-chlorobutyric acid content reached more than 99%. Leave to stand for stratification, collect the product of the lower layer, and repeat the application of the upper layer of water.
Embodiment 3
[0020] The γ-butyrolactone of 86g and the upper strata aqueous phase collected in the example one are put into the flask, keep stirring at room temperature, add 200g of 20% hydrochloric acid dropwise for reaction, after 2h, sample GC analysis, the residue of γ-butyrolactone is less than 1%. The content of γ-chlorobutyric acid reaches more than 99%. Leave to stand for stratification, collect the product of the lower layer, and repeat the application of the upper layer of water. After 10 times of reaction, the content of γ-chlorobutyric acid can still reach more than 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com