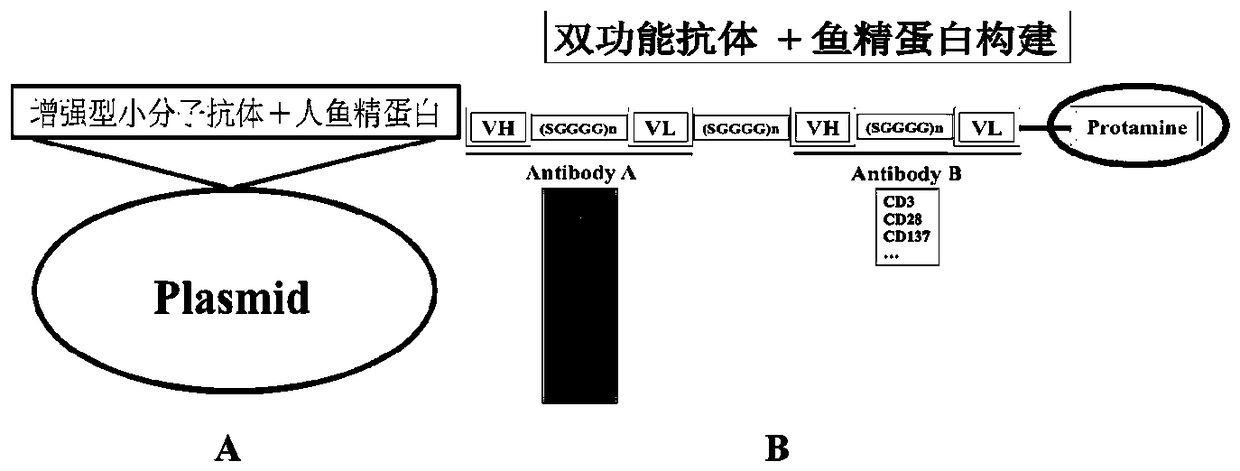
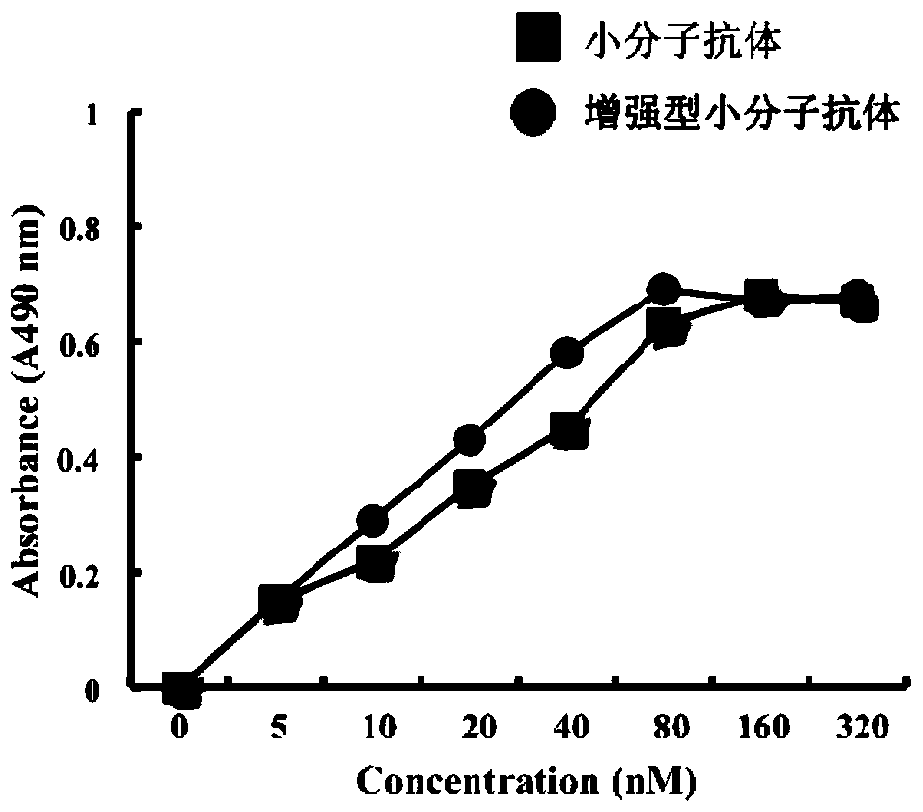
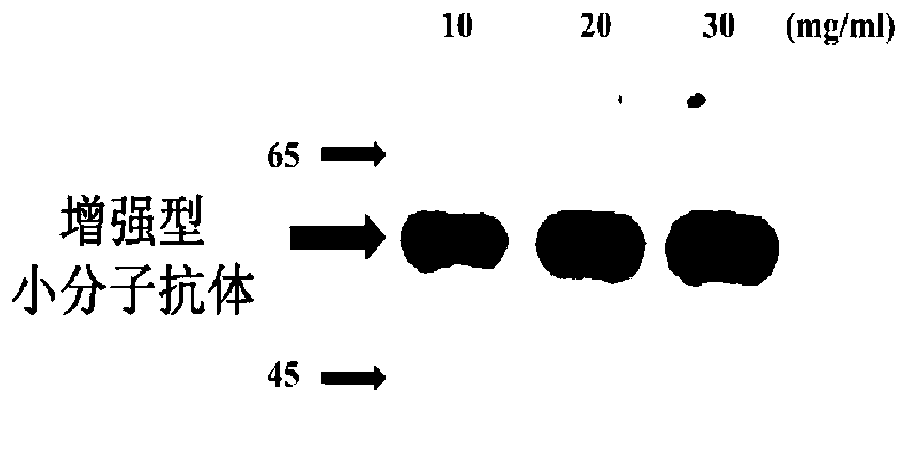
Construction and application of reinforced micromolecular antibody
A small molecule antibody, enhanced technology, applied in the field of biomedicine, can solve the problems of improper dosage, limited application and treatment range, and easy to cause adverse reactions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Embodiment 1 protamine cDNA and amino acid sequence
[0089] human protamine 1 mRNA
[0090] (A total of 304bp, of which the underline is the cDNA encoded amino acid sequence 156bp)
[0091] GACTCACAGCCCACAGAGTTCCACCTGCTCACAGGTTGGCTGGCTCAGCCAAGGTGGTGCCCTGCTCTGAGCATTCAGGCCAAGCCCATCCTGCACC ATGGCCAGGTACAGATGCTGTCGCAGCCAGAGCCGGAGCAGATATTACC GCCAGAGACAAAGAAGTCGCAGACGAAGGAGGCGGAGCTGCCAGACACGGAGGAGAGCCATGAGGTGCTGCCGCCCC AGGTACAGACCGCGATGTAGAAGACACTAA TTGCACAAAATAGCACATCCAAACTCCTGCCTGAGAATGTTACCAGACTTCAAGATCCTCTTGCCACATCTTGAAAATGCCACCATCCAATAAAAATCAGGAGCCTGCTAAGGAACAATGCCGCCTGTCAATAAATGTTGAAAAGTCATCCCAAAAAAAAAAAAAAAAAAA
[0092] Amino acid sequence of human protamine 1 (51aa)
[0093] MARYRCCRSQSRSRYYRQRQRSRRRRRRSCQTRRRAMRCCRPRYRPRCRRH
[0094] human protamine 2 mRNA
[0095] (A total of 651bp, of which the underline is 309bp of cDNA encoded amino acid sequence)
[0096] GGTGGGCAGGCCTCCGCCCTCTCCCCTACTCCAGGGCCCACTGCAGCCTCAGCCCAGGAGCCACCAGATCTCCCAACACC ATGGTCCGATACCGC...
Embodiment 2
[0104] Example 2 Construction and expression of enhanced small molecule antibody ((N-) anti-human CD19 heavy chain variable region antibody+anti-human CD19 light chain variable region antibody (C-)+human protamine 2+His6)
[0105] Basic pattern of enhanced small molecule antibody: Objective Enhanced small molecule antibody: (N-) anti-human CD19 heavy chain variable region antibody + anti-human CD19 light chain variable region antibody (C-) + human protamine 2 + His6 . Basic amplification conditions: 95°C for 5min, followed by 30 cycles of 95°C for 1min, 55°C for 1min, 72°C for 1min, and finally 72°C for 5min. EcoR I and BamH I restriction sites were introduced into the PCR primers. (N-) anti-human CD19 heavy chain variable region antibody+anti-human CD19 light chain variable region antibody (C-)+human protamine 2+His6 cDNA (enhanced small molecule antibody cDNA) was cloned into pMD18T / L19 expression plasmid , the correct sequence was obtained by sequencing.
[0106]The enha...
Embodiment 3
[0109] Example 3 Purification and Identification of Enhanced Small Molecule Antibody
[0110] To purify the expressed protein of interest, the pellet obtained from the medium was washed twice with buffer (20 mM Tris / HCl, pH 8.0, 3% (V / V) Triton X-100 and 1 M urea), and resuspended in 200 ml Denaturing buffer (20mM Tris / HCl, pH 8.0, 8M urea, 150mM NaCl, 10mM imidazole and 10mM 2-mercaptoethanol). Store at 25°C with gentle shaking to ensure complete dissolution of the osmosome. Proteins were purified by immobilized metal-ion-affinity chromatography (IMAC) under denaturing conditions. At 4°C, denature the mixture, 14000g x 20 minutes, and supernatant. Inclusion bodies were dissolved in a lysis buffer at a ratio of 20:1 (7M guanidine hydrochloride, 50mM Tris, 50mM NaCl, 5mM ethyldiaminotetraacetic acid, 50mM dithiothreitol, pH 8.0), 37°C, Incubate for 1 hour. After centrifugation, the supernatant was diluted (20 times) with refolding buffer (50mM Tr-HCl, 50mMNaCl, 0.8mM L-argi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com