Construction and application of an enhanced small molecule antibody
A small molecule antibody, enhanced technology, applied in the field of biomedicine, can solve the problems of limited application and treatment range, easy to cause adverse reactions, improper dose use, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] Example 1 Protamine cDNA and amino acid sequence
[0090] human protamine 1 mRNA
[0091] (A total of 426bp, of which the underline is the cDNA coding sequence 156bp)
[0092] GACTCACAGCCCACAGAGTTCCACCTGCTCACAGGTTGGCTGGCTCAGCCAAGGTGGTGCCCTGCTCTGAGCATTCAGGCCAAGCCCATCCTGCACC ATGGCCAGGTACAGATGCTGTCGCAGCCAGAGCCGGAGCAGATATTACC GCCAGAGACAAAGAAGTCGCAGACGAAGGAGGCGGAGCTGCCAGACACGGAGGAGAGCCATGAGGTGCTGCCGCCCC AGGTACAGACCGCGATGTAGAAGACACTAA TTGCACAAAATAGCACATCCAAACTCCTGCCTGAGAATGTTACCAGACTTCAAGATCCTCTTGCCACATCTTGAAAATGCCACCATCCAATAAAAATCAGGAGCCTGCTAAGGAACAATGCCGCCTGTCAATAAATGTTGAAAAGTCATCCCAAAAAAAAAAAAAAAAAAA
[0093] Human protamine 1 amino acid sequence (51aa)
[0094] MARYRCCRSQSRSRYYRQRQRSRRRRRRSCQTRRRAMRCCRPRYRPRCRRH
[0095] human protamine 2 mRNA
[0096] (A total of 651bp, of which the underlined cDNA coding sequence is 309bp)
[0097] GGTGGGCAGGCCTCCGCCCTCTCCCCTACTCCAGGGCCCACTGCAGCCTCAGCCCAGGAGCCACCAGATCTCCCAACACC ATGGTCCGATACCGCGTGAGGAGCCTGAGCGAACGCTCGCACGAGGT...
Embodiment 2
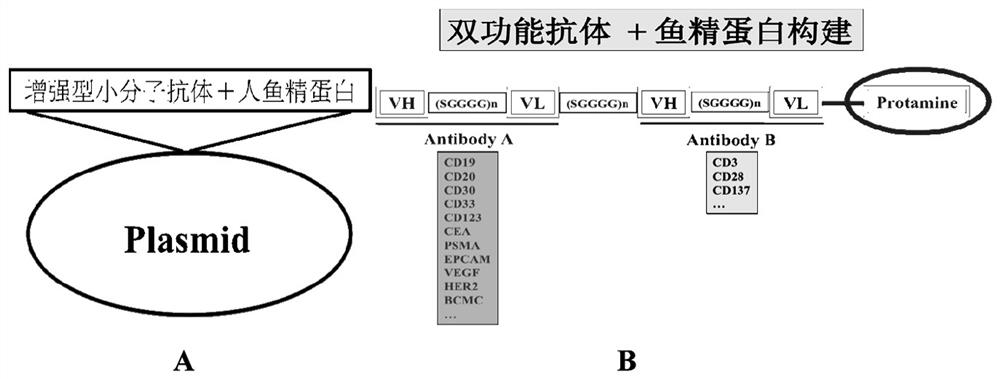
[0105] Example 2 Construction and expression of enhanced small molecule antibody ((N-) anti-human CD19 heavy chain variable region antibody + anti-human CD19 light chain variable region antibody (C-) + human protamine 2 + His6)
[0106] Basic pattern of enhanced small molecule antibody: Objective enhanced small molecule antibody: (N-) anti-human CD19 heavy chain variable region antibody + anti-human CD19 light chain variable region antibody (C-) + human protamine 2 + His6. Basic amplification conditions: 95 o After 5min at C, 30 cycles, 95 o C 1min, 55 o C 1min, 72 o C 1min, last extension 72 o C 5min. EcoR I and BamHI restriction sites were introduced into the PCR primers. (N-) Anti-human CD19 heavy chain variable region antibody + anti-human CD19 light chain variable region antibody (C-) + human protamine 2 + His6 cDNA (enhanced small molecule antibody cDNA) was cloned into pMD18T / L19 expression plasmid, The correct sequence was obtained by sequencing.
[0107] The en...
Embodiment 3
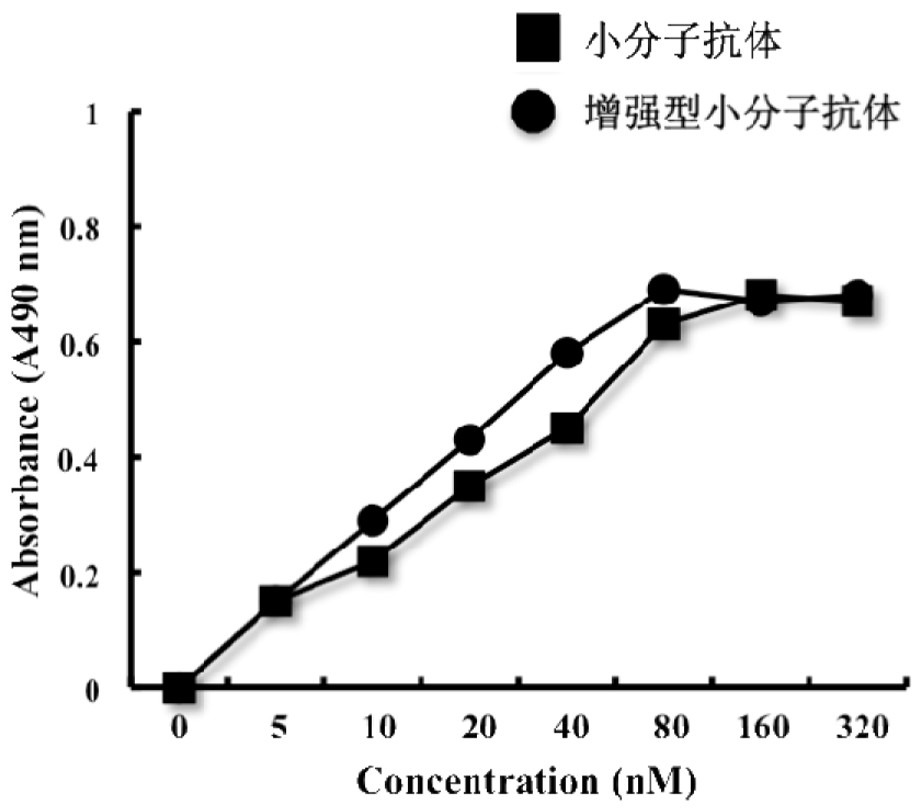
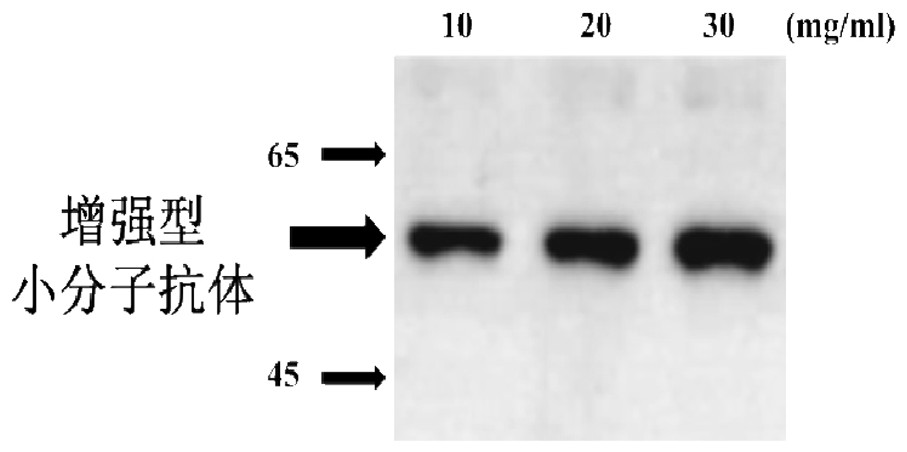
[0110] Example 3 Purification and Identification of Enhanced Small Molecule Antibody
[0111] To purify the expressed target protein, the pellet obtained from the culture medium was washed twice with buffer (20mM Tris / HCl, pH8.0, 3% (V / V) Triton X-100 and 1M urea), and resuspended in 200ml Denaturing buffer (20mM Tris / HCl, pH 8.0, 8M urea, 150mM NaCl, 10mM imidazole and 10mM 2-mercaptoethanol). at 25 o C and store with gentle shaking to ensure complete dissolution of the osmosome. Proteins were purified by immobilized metal-ion-affinity chromatography (IMAC) under denaturing conditions. In; 4 o C, denatured mixture, 14000g×20 minutes, supernatant. Inclusion bodies were dissolved in lysis buffer at a ratio of 20:1 (7M guanidine-butyrine hydrochloride, 50mM Tris, 50mM NaCl, 5mM ethyldiaminotetraacetic acid, 50mM dithiothreitol, pH 8.0), 37 o C, incubate for 1 hour. After centrifugation, the supernatant was diluted (20 times) with refolding buffer (50 mM Tr-HCl, 50 mM NaCl,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com