Fusion TaqDNA polymerase and application thereof
A polymerase and single-strand binding protein technology, applied in the direction of recombinant DNA technology, applications, enzymes, etc., can solve the problems of DNA cross-contamination false positives, unfavorable, long-term, etc., and achieve the effect of stable enzyme activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1 prepares the Taq DNA polymerase of fusion
[0035] We have obtained three genes expressing DNA single-strand binding proteins: HMF, Sac7d, and sso7d, and their sequences can be obtained through existing public access, or SEQ ID NO.4, SEQ ID NO.1, and SEQ ID NO. The wild-type Taq DNA polymerase is removed from the sequence of 2, that is, the remaining sequence after removing the sequence of SEQ ID NO.3. The nucleic acid sequences of HMF, Sac7d, and sso7d were obtained through pre-amplification, gel recovery, and sequencing, and HMF, Sac7d, and sso7d were linked end-to-end with Taq using the ClonExpress Ultra One Step Cloning Kit (Nanjing Novizan Biotechnology Co., Ltd.) respectively in the pET28a vector , to obtain the recombinant gene, transform it into Escherichia coli, shake the bacteria at 37°C for 1 hour (rotating speed 200-250rpm), centrifuge at 5000rpm for 5min, discard 900μl supernatant, resuspend the bacteria in the remaining medium, and spread the ...
Embodiment 2
[0049] The improved Taq DNA polymerase that embodiment 2 detects is directly used in the effect of blood amplification
[0050] The effect of the modified HMF-Taq, Sac7d-Taq, sso7d-Taq and wild-type Taq (sequence shown in SEQ ID NO.3) on blood direct expansion was tested.
[0051] The reaction buffer formula used in the test: 30mM Tris, pH8.3, Mg 2.5mM, KCl 10mM, trehalose 0.5M, reaction enzyme concentration 2U / μl.
[0052] The blood template concentration was 15% of the reaction system. Reaction program: 95°C for 30 sec, 95°C for 5 min, 58°C for 30 sec, 72°C for 30 sec (35 cycles), 72°C for 5 min.
[0053] Primer sequences used for amplification:
[0054] R: CTGCCCGTAGTAGTCGTAATCGTCCTCCAT
[0055] F: AACTGCTGCTTGGCTACAGCGACATCGA
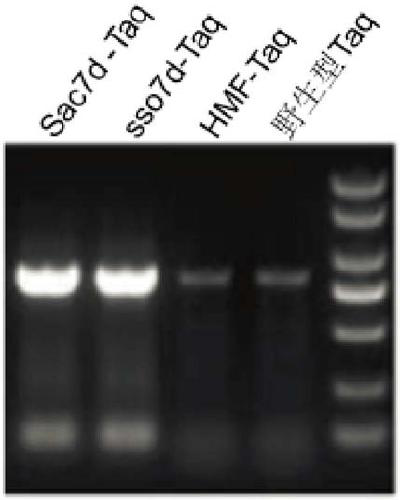
[0056] The PCR products were electrophoresed on a 1% agarose gel to obtain figure 1 The results show that Sac7d-Taq and sso7d-Taq obtained by gene fusion can be well amplified in a system containing 15% blood, while the comparative HMF-Taq and ...
Embodiment 3
[0057] Example 3 Detection of enzyme activities of HMF-Taq, Sac7d-Taq, sso7d-Taq and wild-type Taq in the presence of different concentrations of blood
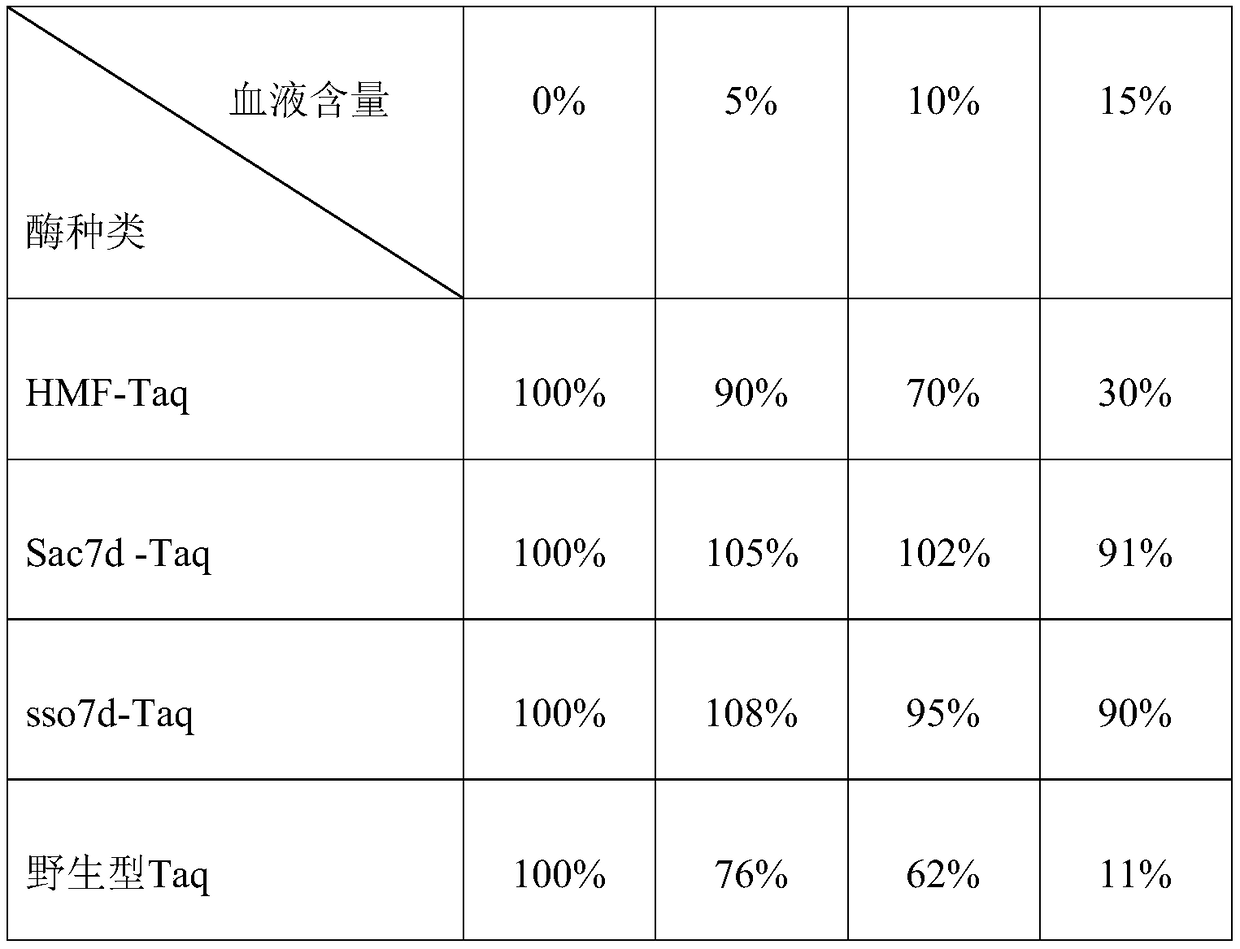
[0058] Four enzymes (HMF-Taq, Sac7d-Taq, sso7d-Taq and wild-type Taq) composed of 3 enzymes prepared in Example 1 and wild-type Taq were mixed with activated calf thymus DNA and P 32 Incubate with labeled dATP, keep it at 70°C for 10 minutes under different volume concentrations of blood inhibition, detect the enzyme activity by detecting the speed of nucleotide infiltration into DNA, and detect it on a microplate reader, the results are shown in Table 1 As shown, it shows that the enzyme activity of Sac7d-Taq and sso7d-Taq obtained by gene fusion is stable in the presence of different blood, and 15% of blood can still maintain more than 90% of the activity, while the wild-type and HMF-Taq activities decrease respectively To 11%, 30%.
[0059] Table 1: 4 kinds of enzymes detect enzyme activity results under blood inhibition ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com