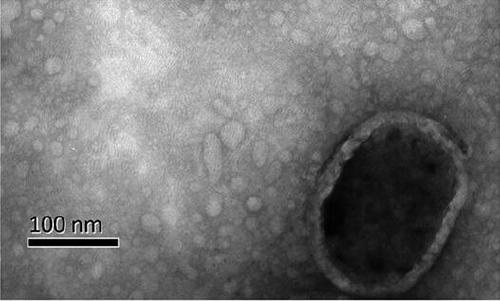
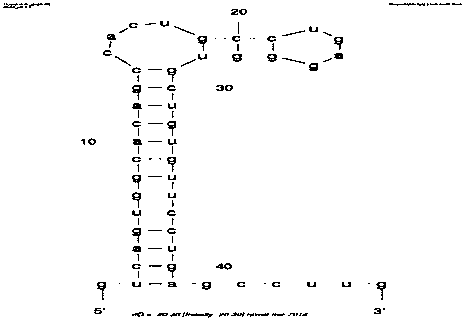
Exosomal biomarker for auxiliary diagnosis of SCA3/MJD and screening and identification method thereof
A technology of secretobiology and auxiliary diagnosis, which is applied in the direction of biochemical equipment and methods, microbial measurement/testing, DNA/RNA fragments, etc. Ineffective treatment and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] In Example 1, the differentially expressed miRNAs related to SCA3 / MJD were screened, and the specific operation process was as follows:
[0034] 1. Sample collection: plasma samples from 12 patients with SCA3 / MJD (including 3 subtypes: 3 cases of type 1, 6 cases of type 2, and 3 cases of type 3) and plasma samples of 12 age- and sex-matched healthy individuals Serve as control group; CSF samples from 12 SCA3 / MJD patients (including 3 subtypes: 3 Type 1, 6 Type 2, 3 Type 3) and 10 age- and sex-matched aneurysm / migraine patients The cerebrospinal fluid samples were used as the control group.
[0035] Plasma samples: Collect 10ml of whole blood from SCA3 / MJD patients and control groups with anticoagulant tubes, centrifuge at 3000rpm at 4°C for 15min, separate the plasma for later use, and complete within 2 hours.
[0036] Cerebrospinal fluid samples: Collect 10-15ml of cerebrospinal fluid from SCA3 / MJD patients and the control group with sterile centrifuge tubes, and comp...
Embodiment 2
[0060] This example uses qPCR to verify differentially expressed miRNAs, wherein the verified miRNAs include novel-mir-9034, novel-mir-7660, novel-mir-5219, novel-mir-8421, novel-mir-769, novel-mir -7014, novel-mir-6628, novel-mir-4682, novel-mir-2738, novel-mir-1643, has-miR-92b-3p, has-miR-486-3p, has-miR-484, has -miR-378g, has-miR-375, has-miR-328-3p, has-miR-3074-5p, has-miR-24-3p, has-miR-206, has-miR-204-5p, has -miR-151a-3p, has-miR-146b-5p, has-miR-142-3p, has-miR-1290, has-miR-1268a, has-miR-122-5p, has-let-7d-3p , the specific operation process of qPCR verification is as follows:
[0061] 1. Sample collection: 18 plasma samples of SCA3 / MJD patients and 16 plasma samples of age- and sex-matched healthy people were used as control group; 10ml of whole blood was collected from SCA3 / MJD patients and control group with anticoagulant tube, 3000rpm 4 Centrifuge at ℃ for 15 minutes, separate the plasma for later use, and complete within 2 hours.
[0062] 2. The extractio...
PUM
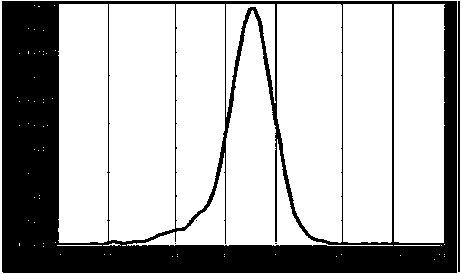
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com