Fusobacterium nucleatum FomA protein vaccine, preparation method and applications thereof
A fusobacterium nucleatum and protein technology, applied in the field of biological vaccines, can solve the problems of the preparation of colon tumor vaccines that have not been seen, and achieve significant protective effects, good stability, and the effects of delaying the occurrence and development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
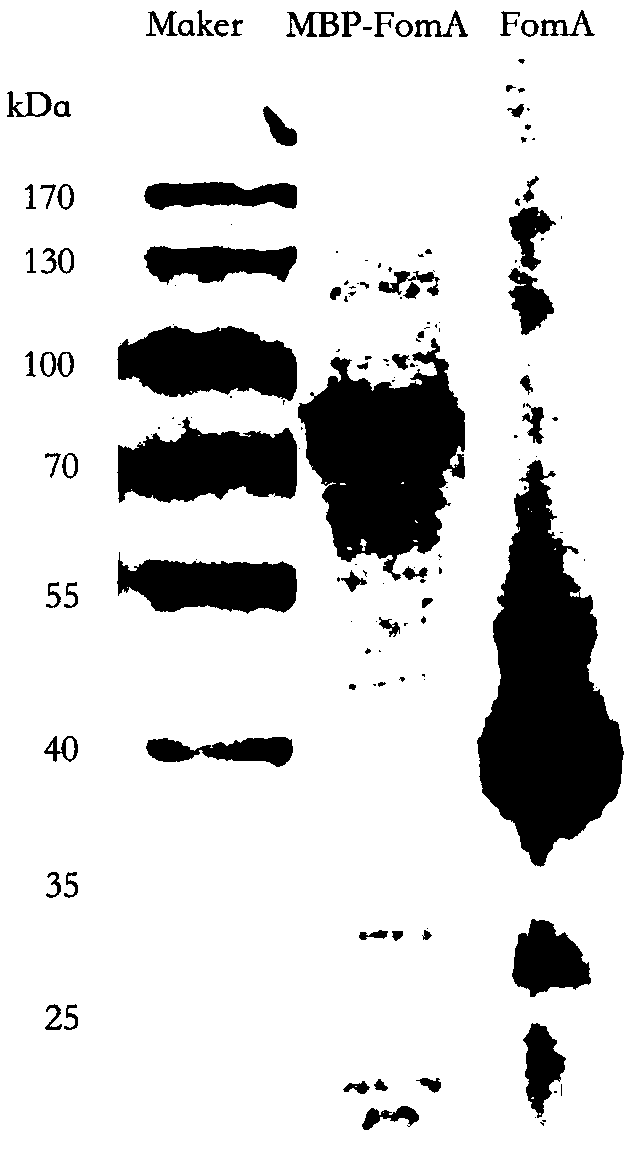
[0040] In a third typical embodiment of the present disclosure, there is provided a method for preparing an anti-colon tumor Fusobacterium nucleatum protein vaccine, the method comprising: connecting the Foma gene with the signal peptide sequence removed and connected with the MBP tag gene to In the expression vector, transform Escherichia coli, induce expression, and purify the FomA recombinant protein containing the MBP tag; then digest the prepared FomA recombinant protein containing the MBP tag to remove the MBP tag protein, and obtain the anti-colon tumor nucleated shuttle bacillus protein vaccine.
[0041] Further, the FomA gene sequence without the signal peptide is shown in SEQ ID NO: 3.
[0042] The FomA recombinant protein antigen prepared in the present disclosure contains MBP tag. It has been verified by experiments that linking the FomA protein sequence with the MBP tag can not only enhance the expression of FomA protein when exogenously expressed, but also improv...
Embodiment 1
[0060] A preparation method of Fusobacterium nucleatum FomA recombinant protein vaccine, comprising the steps of:
[0061] (1) Ligate the Foma gene that removes the signal peptide and connects with the MBP tag gene to the PetDuet-1 carrier to obtain the expression vector, and introduce the expression vector into Escherichia coli to obtain the expression strain; In LB medium, shake culture at 37°C;
[0062] Wherein, the FomA gene sequence is shown in SEQ ID NO: 1, 1-60 bp is the sequence corresponding to the signal peptide, and the sequence shown in SEQ ID NO: 3 is the FomA gene sequence without the signal peptide sequence.
[0063] (2) When the expression strain proliferates until the OD600 is 0.5-0.6, add IPTG with a mass concentration of 0.1% (that is, the IPTG in the culture system is 0.1%), induce expression overnight at 18°C, collect the cells, and lyse to obtain the protein;
[0064] The FomA protein sequence encoded by the FomA gene is shown in SEQ ID NO: 2, wherein 1-...
Embodiment 2
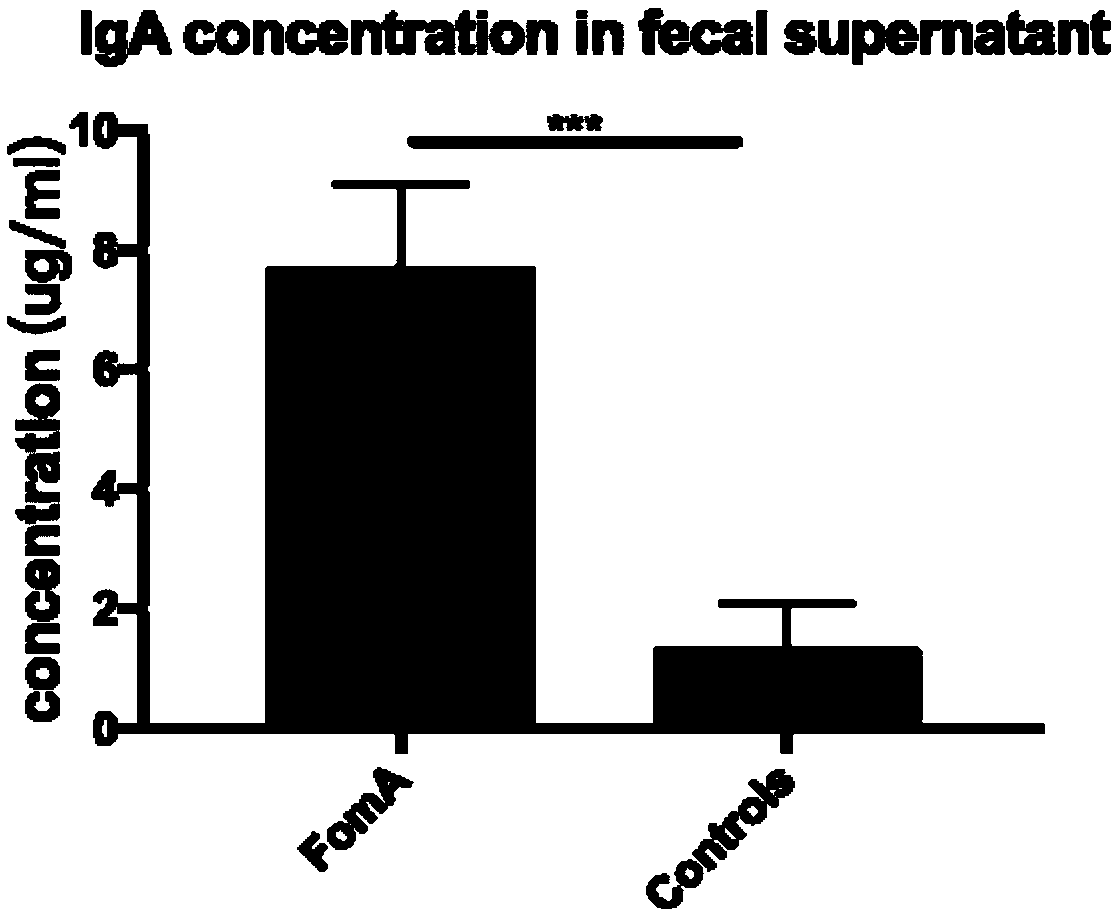
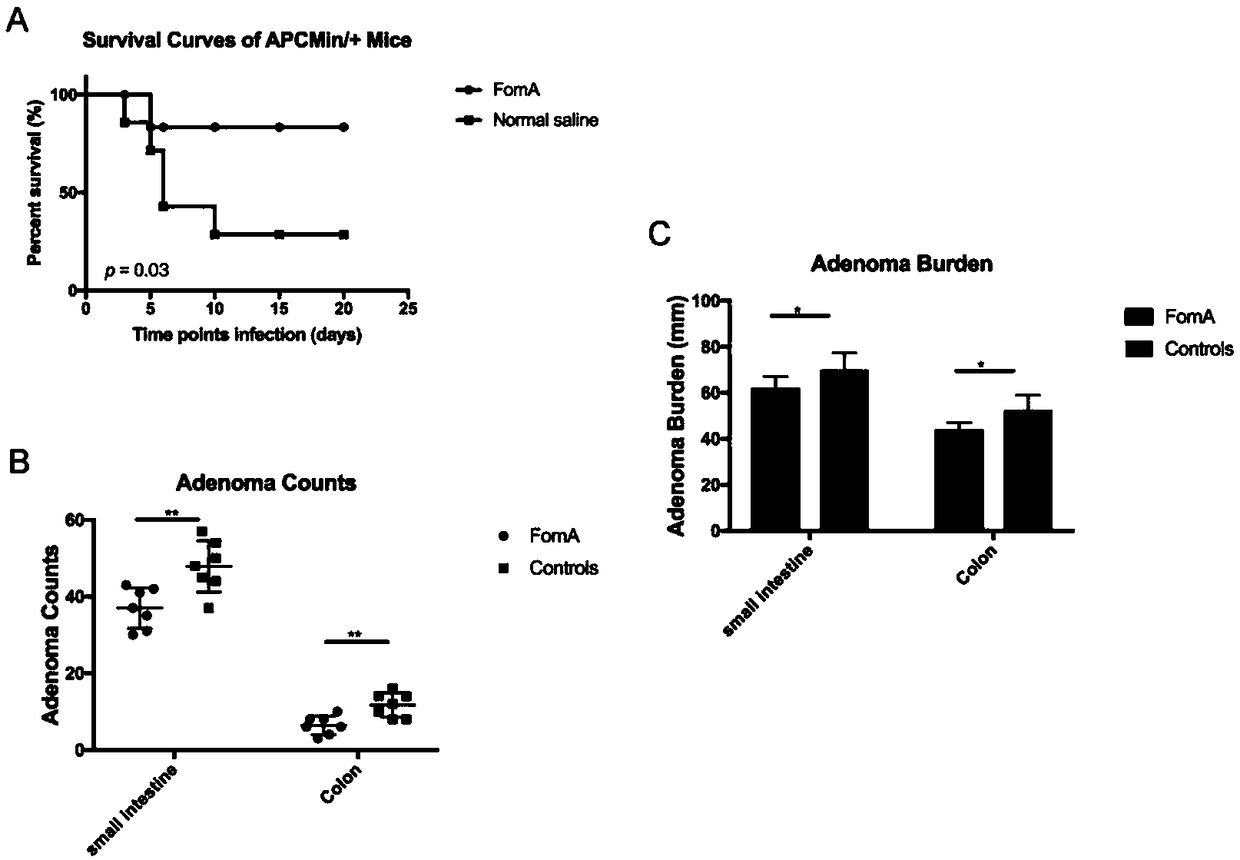
[0070] The Foma recombinant protein vaccine prepared in Example 1 was used to immunize C57BL / 6 mice by gavage, and the control group C57BL / 6 mice were gavaged with normal saline, 50ug protein / mouse, and 7 healthy mice in each group. There was no significant difference in body weight of the mice. The mice were immunized three times with an interval of 7 days. The concentration of Foma-specific IgA antibody in the supernatant of mouse feces was detected 7 days after the last gavage. The concentration of IgA antibody in the fecal supernatant after the immune response was as follows: figure 2 As shown, the average IgA antibody concentration in the feces supernatant of the mice in the FomaA group was 7.71ug / mL, and the average IgA antibody concentration in the feces supernatant of the mice in the control group was 1.30ug / mL.
[0071] The results showed that after three immunizations, the antibody concentration in the feces supernatant of C57BL / 6 mice gavaged with FomA recombinant ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com