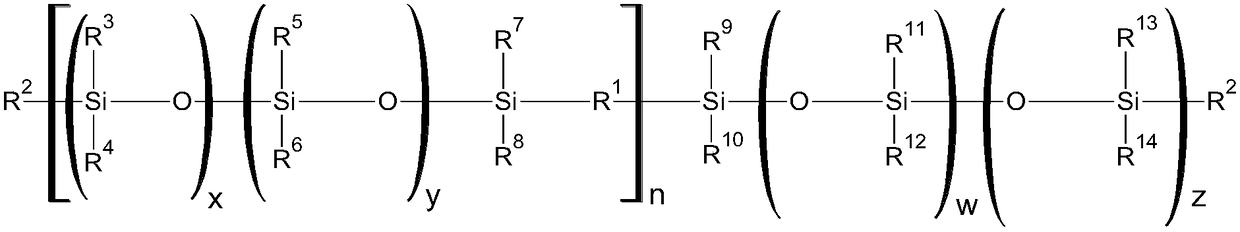
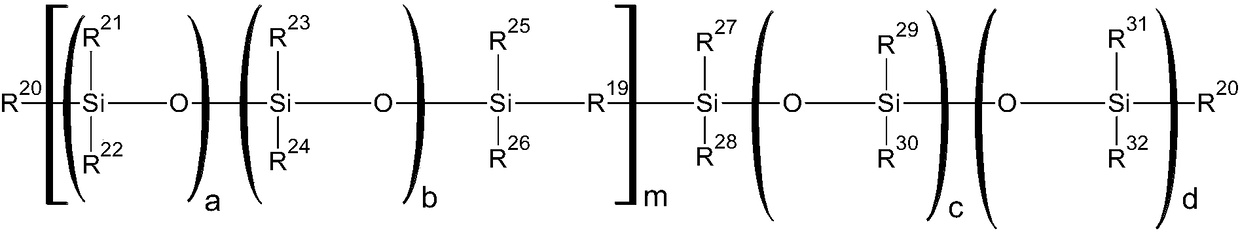
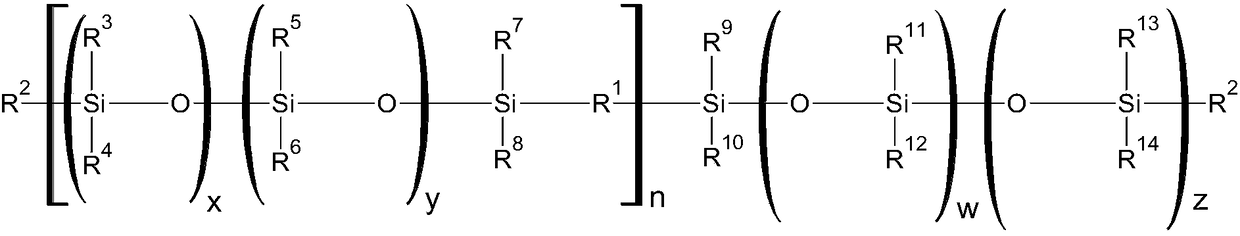
Curable silicone composition and applications and uses thereof
A composition and organosilicon technology, which is applied in the direction of medical preparations containing active ingredients, cosmetics, and dressing preparations, etc., can solve the problems of complex film preparation, high cost, and large-scale and large-scale manufacturing processes.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0161] Embodiment 1: the preparation of component (A-1)
[0162] A norbornene-terminated norbornenyl ethyl block copolymer (A-1) having a molecular weight of 3 kD was synthesized according to the following scheme:
[0163] Under nitrogen atmosphere, 100 mL of toluene and 5-vinylbicyclo[2.2.1]hept-2-ene (128.7 g , 1.07 mol). To this solution was added 0.289 g of Karstedt's catalyst (15 ppm, 2% by weight Pt). The whole apparatus was kept in an oil bath and the reaction temperature was kept at 50°C. 3-Phenyl-1,1,3,3,5-pentamethyltrisiloxane (257.51 g, 0.95 mol) was added dropwise in the dropping funnel over 1 hour. The reaction temperature was then raised to 80 °C and continued until all hydride was consumed. After the hydrosilylation polymerization was complete, unreacted starting materials, volatile compounds and solvents were stripped under reduced pressure. The final product was obtained in quantitative yield as a yellow liquid, decolorized with activated carbon, and the...
Embodiment 2
[0165] Embodiment 2: the preparation of component (A-2)
[0166] A norbornene-terminated phenylmethylsilicone norbornenylethyl block copolymer (component A-2) having a molecular weight of 140 kD was synthesized similarly to the method described in A-1.
[0167] Briefly, 150 mL of toluene and 5-vinylbicyclo[2.2.1]hept-2-ene (94 g, 0.78 mol) were added to a 500 mL three-neck round bottom flask. To this solution was added 0.225 g of Karstedt's catalyst (15 ppm, 2% by weight Pt). 3-Phenyl-1,1,3,3,5-pentamethyltrisiloxane (200 g, 0.74 mol) in a dropping funnel was added dropwise to the reaction mixture at 50° C. over 1 hour. The reaction temperature was then raised to 80 °C and continued until all hydride was consumed. After the hydrosilylation polymerization was complete, unreacted starting materials, volatile compounds and solvents were stripped under reduced pressure. The final product was obtained in quantitative yield as a yellow liquid, which was decolorized with activated...
Embodiment 3
[0169] Embodiment 3: the preparation of component (B-1)
[0170] Hydride terminated phenylmethyl silicone norbornenyl ethyl block copolymer (component B) was synthesized according to the following scheme:
[0171]Under a nitrogen atmosphere, 100 mL of toluene and 5-vinylbicyclo[2.2.1]hept-2-ene (200 g, 1.66mol). To this solution was added 0.529 g of Karstedt's catalyst (15 ppm, 2% by weight Pt). The whole apparatus was kept in an oil bath and the reaction temperature was kept at 50°C. 3-Phenyl-1,1,3,3,5-pentamethyltrisiloxane (506.47 g, 1.87 mol) was added dropwise in a dropping funnel over 1 hour. The reaction temperature was then raised to 80°C and the reaction was continued until all the 5-vinylbicyclo[2.2.1]hept-2-ene was consumed. After the hydrosilylation polymerization was complete, unreacted starting materials, volatile compounds and solvents were stripped under reduced pressure. The final product was obtained in quantitative yield as a yellow liquid, which was de...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com