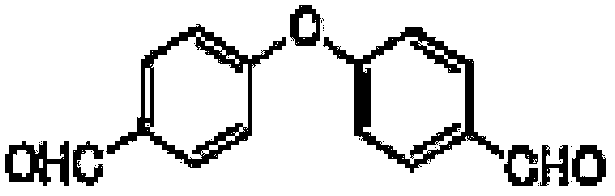
Refining method of 4-(4-formylphenoxy)benzaldehyde
The technology of a formylphenoxy group and a refining method is applied in the refining field of 4-benzaldehyde, can solve the problems that the purity is difficult to reach more than 99.5%, cannot meet the purity of electronic-grade products, etc., and achieves easy recycling, low production cost, The effect of improving quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] A kind of refining method of 4-(4-formylphenoxy)benzaldehyde:
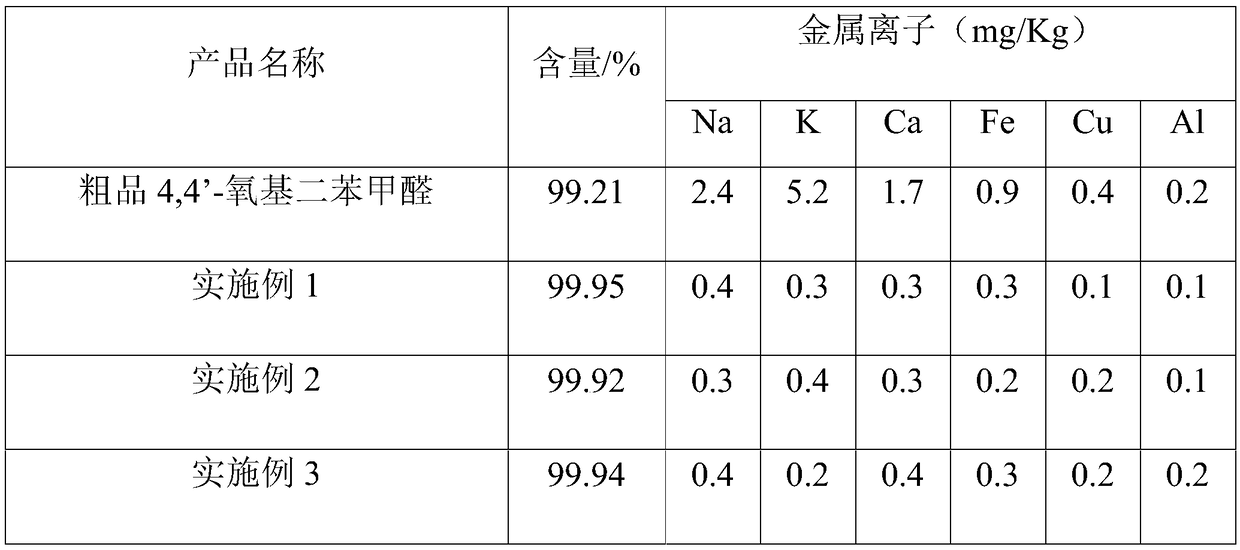
[0048]Step 1. Add 6kg of crude product 4-(4-formylphenoxy)benzaldehyde and 30kg of dichloromethane to a 50L glass reactor equipped with heating mantle, mechanical stirring and reflux condenser, stir to dissolve, then add 0.5wt % sodium hydroxide solution 9Kg, stirred and reacted at 10°C for 30min, stood still for 1h, separated the liquid, transferred the oil layer to a 100L glass reactor, washed with 30kg pure water until neutral, stood still to separate the liquid, and collected the oil phase;
[0049] Step 2. Transfer the obtained oil phase into a 50L glass reactor, add 24mg of potassium carbonate, control the vacuum at -0.099MPa, and the temperature is 50°C, and evaporate the dichloromethane solvent to dryness to obtain a concentrate;
[0050] Step 3: Add 36kg of methanol to the concentrate, heat up to 55°C and stir to dissolve, transfer to a 100L crystallization kettle, keep the temperature at 55°C, add...
Embodiment 2
[0052] A kind of refining method of 4-(4-formylphenoxy)benzaldehyde:
[0053] Step 1. Add 6kg of crude product 4-(4-formylphenoxy)benzaldehyde and 12kg of chloroform to a 50L glass reactor equipped with heating mantle, mechanical stirring and reflux condenser, stir to dissolve, then add 0.2wt % sodium hydroxide solution 3.4Kg, react with stirring at 15°C for 1h, let stand for 1h, separate the liquid, transfer the oil layer to a 100L glass reactor, wash with 30kg of pure water until neutral, let stand to separate the liquid, and collect the oil phase ;
[0054] Step 2. Transfer the obtained oil phase into a 50L glass reaction kettle, add 12mg of sodium carbonate, control the vacuum to -0.08MPa, and the temperature is 30°C, and evaporate the chloroform solvent to dryness to obtain a concentrate;
[0055] Step 3: Add 18kg of ethanol to the concentrate, heat up to 50°C and stir to dissolve, transfer to a 100L crystallization kettle, keep the temperature at 50°C, add 14.4kg of pur...
Embodiment 3
[0057] A kind of refining method of 4-(4-formylphenoxy)benzaldehyde:
[0058] Step 1. Add 6kg of crude product 4-(4-formylphenoxy)benzaldehyde and 24kg of dichloroethane to a 50L glass reactor equipped with heating mantle, mechanical stirring and reflux condenser, stir to dissolve, then add 1wt % sodium hydroxide solution 7.9Kg, stirred and reacted at 30°C for 30min, stood still for 1h, separated the liquid, transferred the oil layer to a 100L glass reactor, washed with 30kg pure water until neutral, stood still to separate the liquid, and collected the oil phase ;
[0059] Step 2. Transfer the obtained oil phase into a 50L glass reaction kettle, add 36mg of sodium hydroxide, control the vacuum at -0.09MPa, the temperature is 40°C, and evaporate the dichloroethane solvent to dryness to obtain a concentrate;
[0060] Step 3: Add 30kg of isopropanol to the concentrate, raise the temperature to 60°C and stir to dissolve, transfer to a 100L crystallization kettle, keep the temper...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com